Summary
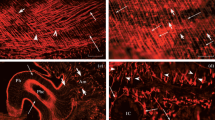
Ultrastructural localization techniques for cholinesterases (ChE) by the Karnovsky method, for monoamines by the technique of Wood, and Tranzer and Richards, and for 5HT by the technique of Wood, were performed on the anterior byssal retractor muscle of Mytilus. Simultaneous ultrastructural localization of 5HT and ChE was obtained by the use of the false neurotransmitter 5,6-DHT, followed by the method of Karnovsky for ChE. The glio-interstitial tissue presents a constant ChE activity, mainly localized on the plasma membrane, even in those processes which accompany tryptaminergic (5HT containing) neurites. Muscle cell membrane also reacts positively to the Karnovsky method. The tryptaminergic neurites themselves do not show any ChE activity; they contain dichromate reactive large (100 nm) dense cored vesicles. The presence of differentiated tryptaminergic neuromuscular junctions, suggested by other authors, is established. It was not possible to distinguish classes of nerve endings by the typology of their vesicular content. It is concluded that one can reasonably plan to study the effect of glial ChE inhibition on the physiology of the tryptaminergic (relaxing) response of the A.B.R.M. to nerve stimulation.
Similar content being viewed by others
References
Albanese A, Butcher LL (1980) Acetylcholinesterase and catecholamine distribution in the locus coeruleus of the rat. Brain Res Bull 5:127–134
Baumgarten HG, Bjorklund A, Holstein AF, Nobin A (1972) Chemical degeneration of indolamine axons in rat brain by 5,6-dihydroxytryptamine. Z Zellforsch 129:256–271
Baumgarten HG, Bjorklund A, Lachenmayer L, Nobin A, Rosengren E (1973) Evidence for the existence of serotonin-, dopamine- and noradrenaline-containing neurons in the gut of Lampetra fluviatilis. Z Zellforsch 141:33–54
Bogusch G (1968) Cholinesteraseaktivität im glatten Penisretraktormuskel der Weinbergschnecke Helix pomatia L. Histochemie 12:345–349
Bogusch G (1972) Zur Histochemie der Cholinesterase im Penisretraktor von Helix pomatia. Z mikrosk-Anat Forsch 85:35–49
Brestkin AP, Brick IL, Grigor'eva GM (1973) Comparative pharmacology of cholinesterases. In: Michelson MJ (ed) Comparative pharmacology. Pergamon Press, Oxford, vol 1
Butcher LL, Talbot K (1978) Acetylcholinesterase in rat nigro-neostriatal neurons-Experimental verification and evidence for cholinergic-dopaminergic interactions in substantia nigra and caudate putamen complex. In: Butcher LL (ed) Cholinergic-monoaminergic interactions in the brain. Academic Press, New York, pp 25–95
Cambridge GW, Holgate JA, Sharpe JA (1959) A pharmacological analysis of the contractile mechanisms of Mytilus muscle. J Physiol 148:451–464
Chiang PK, Bourgeois JG, Bueding E (1972) Histochemical distribution of acetylcholinesterase in the nervous system of the snail, Biomphalaria glabrata. Int J Neurosci 3:47–60
Chubb IW, Hodgson AJ, White GH (1980) Acetylcholinesterase hydrolyzes substance P. Neuroscience 5:2065–2072
Das S, Edwardson JA, Hughes D, McDermott JR (1982) Butyrylcholinesterase (BuChE)-positive glial cells in the pituitary intermediate lobe: evidence for their role in the formation of pituitary colloid and that BuChE is an endopeptidase. J Physiol 327:45P
Davis R, Koelle GB (1978) Electron microscopic localization of the acetylcholinesterase and butyrylcholinesterase in the superior cervical ganglion of the cat. I. Normal ganglion. J Cell Biol 78:785–809
Desmedt JE, La Grutta G (1967) The effect of selective inhibition of pseudo-cholinesterase on the spontaneous and evoked activity of the cat's cerebral cortex. J Physiol 136:20–40
Dhainaut-Courtois N, Engelhardt RP, Dhainaut A (1979) Etude cytophysiologique des systèmes monoaminergiques et cholinergiques des Nereis (Annélides Polychètes). I. Système nerveux périphérique et jonctions neuro-musculaires. Arch Biol 90:225–244
Elekes K, Hiripi L, Nemcsok J (1977) Ultrastructural effects of 6-hydroxydopamine and 5,6-dihydroxytryptamine on the central nervous system of fresh-water mussel, Anodonta cygnea L. Acta Biol Acad Sci Hung 28:259–272
Ellison JP, Olander KW (1972) Simultaneous demonstration of catecholamines and acetylcholinesterase in peripheral autonomic nerves. Am J Anat 135:23–32
Eränkö O (1966) Demonstration of catecholamines and cholinesterases in the same section. Pharmacol Rev 18:353–358
Etcheverry GJ, Zieher LM (1968) Cytochemistry of 5-hydroxytryptamine at the electron microscope level. I. Study of the specificity of the reaction in isolated blood platelets. J Histochem Cytochem 16:162–171
Florey E, Michelson MJ (1973) Occurrence, pharmacology and significance of cholinergic mechanisms in the animal kingdom. In: International encyclopedia of pharmacology and therapeutics, Section 85, vol 1, pp 11–41
Germino NI, Castellano MA, Gerard G (1968) Histochemical detection of several enzymes in Cryptomphallus and Blaptica and its relation to neuromuscular transmission. Comp Biochem Physiol 24:711–716
Gillette R, Pomeranz B (1975) Ultrastructural correlates of interneuronal function in the abdominal ganglion of Aplysia californica. J Neurobiol 6:463–474
Gilloteaux J (1975) Innervation of the anterior byssal retractor muscle (ABRM) in Mytilus edulis L. II. Ultrastructure of the glio-interstitial cells. Cell Tissue Res 161:511–519
Gilloteax J (1977) Innervation du muscle antérieur du byssus (ABRM) de Mytilus edulis L. III. Localisation histochimique des terminaisons nerveuses à 5-hydroxytryptamine. Histochemistry 51:343–351
Gilloteaux J (1978) Innervation du muscle rétracteur antérieur du byssus (ABRM) de Mytilus edulis L. et de Mytilus galloprovincialis LmK. V. Localisation cytochimique d'activiés cholinestérasiques. Histochemistry 55:209–224
Gunn M (1971) Cholinergic mechanisms in the gastro-intestinal tract. J Neurovisc Relat 32:224–240
Hanley MR, Cottrell GA, Emson PC, Fonnum F (1974) Enzymatic synthesis of acetylcholine by a serotonin-containing neurone from Helix. Nature 251:631–633
Hemming FJ, Nicaise G (1982) Environment-dependent development of glial tissue. Brain Res 245:127–130
Hemming FJ, Carpentier P, Nicaise G (1979) Localisation ultrastructurale de cholinestérases dans le tissue glio-interstitiel du muscle rétracteur antérieur (ABRM) de Mytilus edulis (Mollusque, Lamellibranche). Biol Cell 35:29a
Heumann R, Villegas J, Herzfeld DW (1981) Acetylcholine synthesis in the Schwann cell and axon in the giant nerve fibre of the squid. J Neurochem 36:765–768
Imai H, Nakai K, Kamei I, Itakura T, Komai N, Nagai T, Kimura H, Imamoto K, Maeda (1980) Simultaneous detection method of amine and acetylcholinesterase containing nerve fibers in pial vessels. 2. Electron microscopic study. Cell Mol Biol 26:207–210
Ivens C, Mottram DR, Lever JD, Presly R, Howells G (1973) Studies on the acetylcholinesterase (AChE)-positive and-negative autonomic axons supplying smooth muscle in the normal and 6-hydroxydopamine (6-OHDA) treated rat iris. Z Zellforsch 138:211–222
Jacobowitz D, Koelle GB (1965) Histochemical correlations of acetylcholinesterase and catecholamines in post-ganglionic autonomic nerves of the cat, rabbit, and guinea-pig. J Pharmacol Exp Ther 148:225–237
Karnovsky MJ (1964) The localization of cholinesterase activity in rat cardiac muscle by electron microscopy. J Cell Biol 23:217–232
Koelle GB (1963) Cytological distributions and physiological functions of cholinesterase. In: Handbuch der experimentellen Pharmakologie, Vol 15. Springer, Berlin Göttingen Heidelberg, pp 187–298
Korn E (1969) Cholinesterase activity in the tissues of the sanil Helix aspersa. Comp Biochem Physiol 28:923–930
Loe PR, Florey E (1966) The distribution of acetylcholine and cholinesterase in the nervous system and in innervated organs of Octopus dofleini. Comp Biochem Physiol 17:509–522
Majcen Z, Brzin M (1979) Cholinesterases and choline acetyltransferase in the longitudinal muscle of the guinea-pig ileum. Histochemistry 63:295–302
McCaman RE, McCaman MW (1976) Biology of individual cholinergic neurons in the invertebrate central nervous system. In: Goldberg AM, Hanin I (eds) Biology of cholinergic function. Raven Press, New York, pp 485–514
McKenna OC, Rosenbluth J (1973) Myoneural and intermuscular junctions in a molluscan smooth muscle. J Ultrastruct Res 42:434–450
Nicaise G (1969) Detection histochimique de cholinestérases dans les cellules gliales et interstitielles des Doridiens. CR Soc Biol 163:2600–2604
Nicaise G (1970) Cytochimie ultrastructurale des granules glio-interstitiels d'un Gastéropode. Microscopie Electronique (VIIth Intl Congress, Grenoble) 3:677–678
Nicaise G (1973) The gliointerstilial system of molluscs. Int Rev Cytol 34:251–332
Nicaise G, Amsellem J (1983) Cytology of muscle and neuromuscular junction. In: Wilbur KM (ed) The mollusca. Vol 5: Biochemistry and physiology. Academic Press, New York
Pearse AGE (1972) Histochemistry, theoretical and applied, Vol 2. Churchill-Livingstone, Edinburgh and London
Pentreath VW (1976) Ultrastructure of the terminals of an identified 5-hydroxytryptamine-containing neurone marked by intracellular injection of radioactive 5-hydroxytryptamine. J Neurocytol 5:43–61
Peretz B, Estes J (1974) Histology and histochemistry of the peripheral neural plexus in the Aplysia gill. J Neurobiol 5:3–20
Satchell DG, Twarog BM (1978) Identification of 5-hydroxytryptamine (serotonin) released from the anterior byssus retractor muscle of Mytilus californianus in response to nerve stimulation. Comp Biochem Physiol 59:81–86
Sathananthan AH (1976) Degeneration of monoamine nerves in anterior byssus retractor muscles of Mytilus induced by 5,6-dihydroxytryptamine. Cell Tissue Res 172:425–429
Shkolnik LJ, Schwartz JH (1980) Genesis and maturation of serotonergic vesicles in identified giant cerebral neuron of Aplysia. J Neurophysiol 43:929–944
Silver A (1974) The biology of cholinesterases. Frontiers of Biology. North-Holland, Amsterdam Oxford, Vol 36
Tauc L (1977) Transmitter release at cholinergic synapses. In: Cottrell GA, Usherwood PNR (eds) Synapses. Blackie, Glasgow, pp 64–78
Tauc L (1982) Nonvesicular release of neurotransmitter. Physiol Rev 62:857–893
Taxi J, Gautron J (1969) Données cytochimiques en faveur de l'existence de fibres nerveuses sérotoninergiques dans le coeur de l'Aplysie, Aplysia californica. J Microsc 8:627–636
Teravainen H (1969) Ultrastructural distribution of cholinesterase activity in the ventral nerve cord of the earthworm, Lumbricus terrestris. Histochemie 18:177–190
Thompson EB, Schwartz JH, Kandel ER (1976) A radioautographic analysis in the light and electron microscope of identified Aplysia neurons and their processes after intrasomatic injection of L-3H fucose. Brain Res 112:251–281
Thuneberg L (1982) Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol 71:1–130
Thureson-Klein A, Klein RL, Yen SS (1973) Ultrastructure of highly purified sympathetic nerve vesicles: correlation between matrix density and norepinephrine content. J Ultrastruct Res 43:18–35
Trandaburu T (1972) Comparative observations on AChE distribution in pancreas of some amphibians, reptiles and birds, with special reference to the islets of Langerhans. Histochemie 32:271–279
Tranzer JP, Richards JG (1976) Ultrastructural cytochemistry of biogenic amines in nervous tissue: methodologic improvements. J Histochem Cytochem 24:1178–1193
Treherne JE, Smith DS (1965) The metabolism of acetylcholine in the intact central nervous system of an insect (Periplaneta americana L.). J Exp Biol 43:441–454
Twarog BM (1954) Responses of a molluscan smooth muscle to acetylcholine and 5-hydroxytryptamine. J Cell Comp Physiol 44:141–163
Twarog BM (1976) Aspects of smooth muscle function in molluscan catch muscle. Physiol Rev 56:829–838
Twarog BM, Muneoka Y, Ledgere M (1977) Serotonin and dopamine as neurotransmitters in Mytilus: block of serotonin receptors by an organic mercurial. J Pharmacol Exp Ther 201:350–356
Villegas J (1975) Characterization of acetylcholine receptors in the Schwann cell membrane of the squid nerve fibre. J Physiol 249:679–689
Villegas J (1978a) Cholinergic systems in axon-Schwann cell interactions. Trends Neurosci 1:66–69
Villegas J (1978b) Acetylcholine mediated axon-Schwann cell relationship in the squid nerve fiber. In: Jenden DJ (ed) Cholinergic mechanism and psychopharmacology. Plenum Press, New York, pp 387–399
Villegas GM, Villegas J (1974) Acetylcholinesterase localization in the giant nerve fiber of the squid. J Ultrastruct Res 46:149–163
Wallace BG (1981) Distribution of AChE in cholinergic and non-cholinergic neurons. Brain Res 219:190–195
Watts JA, Pierce SK (1978) Acetylcholinesterase: a useful marker for the isolation of sarcolemma from the bivalve (Modiolus demissus demissus) myocardium. J Cell Sci 34:193–208
Winners W, Neef J, von Wachtendonk D (1978) Distribution of cholinesters and cholinesterases in haemolymphs and smooth muscles of molluscs. Comp Biochem Physiol C 61:121–132
Wood JG (1965) Electron microscopic localization of 5-hydroxytryptamine (5-HT). Texas Reports Biol Med 4:828–837
Wood JG (1966) Electron microscopic localization of amines in central nervous tissue. Nature 209:1131–1133
Wood JG (1967) Cytochemical localization of 5-hydroxytryptamine (5HT) in the central nervous system. Anat Rec 157:343–344
Wood JG, Barrnett RJ (1964) Histochemical demonstration of norepinephrine at a fine structural level. J Histochem Cytochem 12:197–209
Zs.-Nagy I (1964) A histochemical study of cholinesterase on the adductor muscle of the fresh water mussel (Anodonta cygnea L.). Ann Biol Tihany 31:153–157
Author information
Authors and Affiliations
Additional information
Supported by the C.N.R.S., L.A. 040 244, and post-doctoral fellowship to R. Aramant.
Rights and permissions
About this article
Cite this article
Hemming, F.J., Aramant, R. & Nicaise, G. Simultaneous ultrastructural localization of serotonin and cholinesterases in Mytilus byssal retractor muscle (A.B.R.M.). Histochemistry 77, 495–510 (1983). https://doi.org/10.1007/BF00495804
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00495804