Abstract
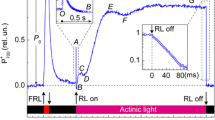
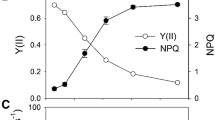
A study of pea plants grown at different light intensities has been made. Using a leaf oxygen electrode, it was shown that plants grown under low light intensities had lower saturated rates of photosynthesis than high-light-grown plants however, at low light intensities the photosynthetic rates were similar for both types of plants. State 1- State 2 transitions have been monitored with attached leaves using a modulated fluorescence technique. It is shown that peas grown under low light intensities (20 W m-2) had a faster State 1 to State 2 transition when compared with medium-(50 W m-2) and high-(70 W m-2) light-grown plants. Measurement of fast-fluorescence-induction curves in the absence of 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU) have shown that low-light plants are, when in State 1, more effective at using Photosystem-two (PSII) light to reduce their plastoquinone pool than high-light plants. Transition from State 1 to State 2 for all plants led to a decrease in the reduction level of the plastoquinone pool inidcating that the transition had increased electron flow through Photosystem one (PSI) relative to PSII. Analyses of fast fluorescence induction in the presence of DCMU indicate that low-light-grown plants have a higher PSII-α/PSII-β ratio than high-light-grown plants. Such a difference is in line with the increase in the PSII/PSI ratio of low-light plants and is reflected in their high chlorophyll b/chlorophyll a ratio and their larger appressed to non-appressed thylakoid-membrane areas. It is suggested that these two latter factors give rise to the faster State 1 - State 2 transitions in low-light plants.
Similar content being viewed by others
Abbreviations
- DCMU:
-
3-(3′,4′-dichlorophenyl)-1,1-dimethylurea
- PSI:
-
Photosystem one
- PSII:
-
Photosystem two
References
Allen, J.F., Bennett, J., Steinback, K.E., Arntzen, C.J. (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature (London) 291, 21–25
Anderson, J.M. (1981) Consequences of spatial separation of photosystem 1 and Photosystem 2 in thylakoid membranes of higher plant chloroplasts. FEBS Lett. 124, 1–10
Anderson, J.M. (1982) The significance of grana stacking in chlorophyll b containing chloroplasts Photobiochem. Photobiophys. 3, 225–241
Andersson, B., Anderson, J.M. (1980) Lateral heterogeneity in the distribution of chlorophyll protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta 593, 427–440
Arnon, D.L. (1949) Copper enzymes in isolated chloroplasts. Polyphenol-oxidase in Beta vulgaris. Plant Physiol. 24, 1–15
Arntzen, C.J. (1978) Dynamic structural features of chloroplast lamellae. In: Current topics in bioenergetics, vol. 8: Photosynthesis, pt. B, pp. 111–160, Rao Sanadi, D., Vernon L.P., eds. Academic Press, New York London
Barber, J. (1976) Ionic regulation in intact chloroplasts and its effect on primary photosynthetic processes. In: The intact chloroplast, vol. 1: Topics in photosynthesis, pp. 88–134, Barber, J., ed. Elsevier, Amsterdam
Barber, J. (1982a) Influence of surface charges on thylakoid structure and function. Annu. Rev. Plant Physiol. 33, 261–295
Barber, J. (1982b) The control of membrane organisation by electrostatic forces. Bioscience Rep. 2, 1–13
Barber, J., Horler, D.N.H., Chapman, D.J. (1981) Photosynthetic pigments and efficiency in relation to the spectral quality of absorbed light. In: Plants and the daylight spectrum, pp. 341–354, Smith, H., ed. Academic Press, New York London
Bennett, J. (1979). Chloroplast phosphoproteins. The protein kinase of thylakoid membranes is light dependent. FEBS Lett. 103, 342–344
Bennett, J., Steinback, K.E., Arntzen, C.J. (1980) Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membranes. Proc. Natl. Acad. Sci. USA 77, 5253–5257
Bennoun, P. (1974) Correlation between State 1 and State II in algae and the effect of magnesium on chloroplasts. Biochim. Biophys. Acta 368, 141–147
Bjorkmann, O. (1981) Responses to different quantum flux densities. In: Encyclopedia of plant physiology, N.S., vol. 12A: Physiological plant ecology I, pp. 57–107, Lange, O.K., Nobel, P.S., Osmond, C.B., Ziegler, H., eds. Springer, Berlin Heidelberg New York
Boardman, N.K. (1977) Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 28, 355–377
Boardman, N.K., Bjorkman, O., Anderson, J.M., Goodchild, D.J., Thorne, S.W. (1974) Photosynthetic adaptation of higher plants to light intensity. Relationship between chloroplast structure, composition of the photosystems and photosynthetic rates. In: The proceedings of the 3rd international congress on photosynthesis, vol. 3, pp. 1809–1823, Avron, M., ed. Elsevier Amsterdam
Bonaventura, C., Myers, J. (1969), Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim. Biophys. Acta 189, 366–383
Bowes, J.M., Horton, P. (1982) The effect of redox potential on the kinetics of fluorescence induction in PSII particles from Phormidium laminosum. Sigmoidicity, energy transfer and the slow phase. Biochim. Biophys. Acta 680, 127–133
Bradbury, M., Baker, N.R. (1981) Analysis of the slow phase of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron-acceptors and fluorescence emission from photosystems I and II. Biochim. Biophys. Acta 635, 542–551
Chow, W.S., Telfer, A., Chapman, D.J., Barber, J. (1981) State 1 — State 2 transition in leaves and its association with ATP induced chlorophyll fluorescence quenching. Biochim. Biophys. Acta 638, 60–68
Delieu, T., Walker, D.A. (1981) Polarographic measurements of photosynthetic oxygen evolution by leaf dises. New Phytol. 89, 165–178
Grumbach, K.H., Lichtenthaler, H.K. (1982) Chloroplast pigments and their biosynthesis in relation to light intensity. Photochem. Photobiol. 35, 209–212
Horton, P., Allen, J.F., Black, M.T., Bennett, J. (1981) Regulation of phosphorylation of chloroplast membranes polypeptides by the redox state of plastoquinone. FEBS Lett. 125, 193–196
Horton, P., Black, M.T. (1980) Activation of adenosine 5′triphosphate induced quenching of chlorophyll fluorescence by reduced plastoquinone. The basis of State 1 — State 2 transitions in chloroplasts. FEBS Lett. 119, 141–144
Lichtenthaler, H.K., Büschmann, C., Döll M., Fietz, H.J., Back, T., Kozel, U., Meier, D., Rähmsdorf, U. (1981) Photosynthetic activity, chloroplast ultrastructure, and leaf characteristic of high-light and low-light plants and of sun and shade leaves. Photosynthesis Res. 2, 115–141
Malkin, S., Armond, P.A., Mooney, H.A., Fork, D.C. (1981) Photosystem II photosynthetic unit size from fluorescence induction in leaves. Correlation to photosynthetic capacity. Plant Physiol. 67, 570–579
Meier, D., Lichtenthaler, H.K. (1981) Ultrastructural development of chloroplasts in radish seedlings grown at high and low light conditions and in the presence of the herbicide bentazon. Protoplasma 107, 195–207
Melis, A., Akoyunoglou, G. (1977) Development of the two heterogeneous photosystem II units in etiolated bean seedlings. Plant Physiol. 59, 1156–1160
Melis, A., harvey, G.W. (1981) Regulation of photosystem stoichiometry, Chl a and Chl b content and relation to chloroplast ultrastructure. Biochim. Biophys. Acta 637, 138–145
Melis, A., Homann, P.H. (1978) A selective effect of Mg2+ on the photochemistry at one type of reaction centre in photosystem II of chloroplasts. Arch. Biochim. Biophys. Acta 190, 523–530
Melis, A., Thielen, A.P.G.M. (1980) The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim. Biophys. Acta 589, 275–286
Myers, J. (1971) Enhancement studies in photosynthesis. Annu. Rev. Plant Physiol. 22, 289–312
Telfer, A., Barber, J. (1981) ATP dependent State 1 - State 2 changes in isolated pea thylakoids. FEBS Lett. 129, 161–165
Ried, A., Reinhardt, B. (1977) Distribution of excitation energy between photosystem I and photosystem II in red algae. II. Kinetics of the transition between state 1 and state 2. Biochim. Biophys. Acta 446, 25–35
Williams, W.P. (1977) The two photosystems and their interactions. In: Primary processes in photosynthesis, vol. 2: Topics in photosynthesis, Barber, J. ed. Elsevier, Amsterdam
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hodges, M., Barber, J. Photosynthetic adaptation of pea plants grown at different light intensities: State 1 - State 2 transitions and associated chlorophyll fluorescence changes. Planta 157, 166–173 (1983). https://doi.org/10.1007/BF00393651
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00393651