Summary
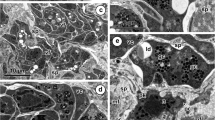
The yolk platelets ofXenopus laevis have been studied by thin-section and freeze-fracture electron microscopy to characterize the boundary membrane during yolk formation. Throughout vitellogenesis, large yolk platelets are in close contact with smaller nascent yolk organelles. Two types of primordial yolk platelets (I and II) have been discriminated. After membrane fusion these precursors can be completely incorporated into the main body of existing platelets, numerous yolk crystals then merge and form one uniformly stratified core. Lipid droplets are tightly attached to the membrane at all developmental stages of yolk platelets. A direct connection of endoplasmic reticulum to the membranes of yolk platelets was not observed. On freezeetching replicas, yolk-platelet membranes present fracture faces with intramembranous particles (IMP) of various sizes and a heterogeneous distribution of approximately 200–600 IMP/μm2 at the E face, and 1200–2100 IMP/μm2 at the P face. Again, this presentation of the membrane exhibits neither anastomoses to the endoplasmic reticulum, nor caveolae that exclude the uptake of yolk-containing vesicles into these yolk organelles. Proteinaceous yolk platelets tend to fracture along their periphery through the superficial layers.
Similar content being viewed by others
References
Benavente R, Krohne G, Franke WW (1985) Cell type-specific expression of nuclear lamina proteins during development ofXenopus laevis. Cell 41: 177–190
Bluemink JG, Hage WJ, van der Hoef MH, Dictus WJAG (1983) Freeze-fracture electron microscopy of membrane changes in progesterone-induced maturing oocytes and eggs ofXenopus laevis. Eur J Cell Biol 31: 85–93
Branton D, Bullivant S, Gilula NB, Karnovsky MJ, Moor H, Mühlethaler K, Northcote DH, Packer L, Satir B, Satir P, Speth V, Staehelin LA, Steere R, Weinstein R (1975) Freeze etching nomenclature. Science 190: 54–56
Dumont JN (1972) Oogenesis inXenopus laevis (Daudin) I. Stages of oocyte development in laboratory maintained animals. J Morphol 136: 153–180
Favard P, Favard-Séréno C (1969) Electron microscope study of polysaccharides in the amphibian oocytes. J Submicrosc Cytol 1: 91–111
Ibrahim KB (1980) Estimation of sodium and potassium in yolk platelets of totad (Bufo bufo) oocytes by electron micro-probeX-Ray analysis. J Physiol (Lond) 305: 91–92
Imoh H (1982) Behaviour of annulate lamellae during the maturation of oocytes in the newt,Cynops pyrrhogaster. J Embryol Exp Morphol 70: 153–169
Karasaki S (1963) Studies on amphibian yolk. 5. Electron microscopic observations on the utilization of yolk platelets during embryogenesis. J Ultrastruct Res 9: 225–247
Kessel RG, Ganion LR (1980a) Electron microscopic and autoradiographic studies on vitellogenesis inNecturus maculosus. J Morphol 164: 215–233
Kessel RG, Ganion LR (1980b) Cytodifferentiation in theRana pipiens oocyte. VI. The origin and morphogenesis of primary yolk precursor complexes. J Submicrosc Cytol 12: 647–654
Kress A (1982) Ultrastructural indications for autosynthetic proteinaceous yolk formation in amphibian oocytes. Experientia 38: 761–771
Lange RH, Richter H-P, Riehl R, Zierold K, Trandaburu T, Magdowski G (1983) Lipovitellin-phosvitin crystals with orthorhombic features: thin-section electron microscopy, gel electrophoresis, and microanalysis in teleost and amphibian yolk platelets and in comparison with other vertebrates. J Ultrastruct Res 83: 122–140
Ling GN (1984) Counterarguments against alleged proof of the Na−K pump in studies of K+ and Na+ distributions in amphibian eggs. Physiol Chem 16: 293–305
Mes-Hartree M, Armstrong JB (1976) Lipid composition of developingXenopus laevis embryos. Can J Biochem 54: 578–582
Richter H-P (1987) Membranes during yolk-platelet development in oocytes of the toadBufo marinus Roux's Arch Dev Biol 196: 367–371
Richter H-P, Jung D, Passow H (1984) Regulatory changes of membrane transport and ouabain binding during progesterone-induced maturation ofXenopus oocytes. J Membr Biol 79: 203–210
Robertson N (1979) Carbohydrate content of isolated yolk platelets from early developmental stages ofXenopus laevis. Cell Differ 8: 173–185
Wall DA, Meleka I (1985) An unusual lysosome compartment involved in vitellogenin endocytosis byXenopus oocytes. J Cell Biol 101: 1651–1664
Wall DA, Patel S (1987) Multivesicular bodies play a key role in vitellogenin endocytosis byXenopus oocytes. Dev Biol 119: 275–289
Wallace RA (1963) Studies on amphibian yolk. IV. An analysis of the main-body components of yolk platelets. Biochim Biophys Acta 74: 505–518
Wallace RA (1970) Studies on amphibian yolk. IX.Xenopus vitellogenin. Biochim Biophys Acta 215: 176–183
Ward RT (1978a) The origin of protein and fatty yolk inRana pipiens. III. Intra-mitochondrial and primary vesicular yolk formation in frog oocytes. Tissue Cell 10: 515–524
Ward RT (1978b) The origin of protein and fatty yolk inRana pipiens. IV. Secondary vesicular yolk formation in frog oocytes. Tissue Cell 10: 525–534
Wiley HS, Wallace RA (1981) The structure of vitellogenin. Multiple vitellogenins inXenopus laevis give rise to multiple forms of the yolk proteins. J Biol Chem 256: 8626–8634
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richter, H.P. Yolk organelles and their membranes during vitellogenesis ofXenopus oocytes. Roux's Arch Dev Biol 198, 92–102 (1989). https://doi.org/10.1007/BF02447744
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02447744