Abstract
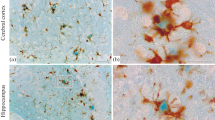
Integrins belonging to different subfamilies can be identified immunohistochemically in cerebral amyloid plaques. Monoclonal antibodies against the VLA family β1-integrins show staining of the corona of classical amyloid plaques for β1, α3 and α6. Immunostaining reveal also the presence of collagen and laminin in the corona. Activated microglial cells in classical plaques strongly express receptors belonging to the LeuCAM family (β2 integrins). The ligands ICAM and activated complement C3 are found in both amorphous and classical plaques. Vitronectin receptor (αv) is found in glial cells in classical plaques but its ligand vitronectin is seen in both amorphous and classical plaques. The data presented here demonstrate the presence of different cellular and substrate adhesive molecules (intregrins) and their ligands in classical plaques. The findings suggest that amyloid plaques show signs of regeneration and tissue remodelling.
Similar content being viewed by others
References
Abraham CR, Selkoe DJ, Potter H (1988) Immunochemical identification of there serine protease inhibitor α1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell 52: 487–501
Akiyama H, McGeer PL (1991) Brain microglia constitutively express β-2 integrins. J Neuroimmunol 30: 81–93
Akiyama H, Kawamata T, Dedhar S, McGeer PL (1990) Immunohistochemical localization of vitronectin, its receptor and beta-3 integrin in Alzheimer brain tissue. J Neuroimmunol 32: 19–28
Amiot M, Huet S, Azogui D, Dastot H, Bernard A, Boumsell A (1988) CDw 29 molecules from the monocyte surface are required for CD3 induced T cell activation. In: Du Pont B (ed) Immunobiology of HLA, vol II. Immunogenetics and histocompatibility. Springer, Berlin Heidelberg New York, pp 556–559
Bodmer S, Podlisny M, Selkoe DJ, Fontana A (1990) Transforming growth factor-beta bound to soluble derivates of the beta amyloid precursor protein of Alzheimer's disease. Biochem Biophys Res Comm 171: 890–897
Bouman L (1934) Senile plaques. Brain 57: 128–142
Breen KC (1992) APP-collagen interaction is mediated by a heparin bridge mechanism. Mol Chem Neuropathol 16: 109–121
Bueé L, Hof PR, Roberts DD, Délacourte A, Morrison JH, Fillit HM (1992) Immunohistochemical identification of thrombospondin in normal human brain and in Alzheimer's brains. Am J Pathol 141: 783–788
Coria F, Cartano E, Prelli F, Larrondo-Lilli M, van Duinen S, Shelanski ML, Frangione B (1988) Isolation and characterization of amyloid P-component from Alzheimer's disease and other cerebral amyloidosis. Lab Invest 58: 454–458
Dustin ML, Rothlein R, Bhan AR, Dinarello CA, Springer TA (1986) Induction by IL-1 and interferon, tissue distribution, biochemistry and function of a natural adherance molecule (ICAM-1). J Immunol 137: 245–254
Eikelenboom P, Stam FC (1982) Immunoglobulins and complement factors in senile plaques. An immunohistochemical study. Acta Neuropathol (Berl) 57: 239–242
Eikelenboom P, Hack CE, Rozemuller JM, Stam FC (1989) Complement activation in amyloid plaques in Alzheimer's dementia. Virchows Arch [B] 56: 259–262
Eikelenboom P, Rozemuller JM, Kraal G, Stam FC, McBride PA, Bruce ME, Fraser H (1991) Cerebral amyloid plaques in Alzheimer's disease but not in scrapie — affected mice are closely associated with a local inflammatory process. Virchows Arch [B] 60: 329–331
Eikelenboom P, Hack CE, Kamphorst W, Rozemuller JM (1992) Distribution pattern and functional state of complement proteins and α1-antichymotrypsin in cerebral β/A4 deposits in Alzheimer's disease. Res Immunol 143: 617–620
Fischer O (1907) Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmäßige Veränderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiatr Neurol 22: 361–372
Frohman EM, Frohman TC, Gupta S, de Fougerolles A, van de Noort (1991). Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer's disease. J Neurol Sci 106: 105–111
Geddes JW, Monaghan DT, Cotman CW, Lott IT, Kim RC, Chui HC (1985) Plasticity of hippocampal circuitry in Alzheimer's disease. Science 230: 1179–1181
Giltay JC, Brinkman HJ, Modderman PW, van dem Borne AE, van Mourik JA (1989) Human vascular endothelial cells express a membrane complex immunochemically indistinguishable from the platelet VLA-2 glycoprotein Ia-IIa complex. Blood 73: 1235–1241
Glenner GG, Wong CW (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885–890
Hack CE, Paardekoper J, Smeenk RJT, Abbink J, Eerenberg AJM, Nuyens JH (1988) Disruption of the internal thioexter bond of the third component of the complement (C3) results in the exposure of neodeterminants also present on activation products of C3. J Immunol 141: 1602–1609
Heino J, Ignotz R, Hemler ME, Crouse C, Massagué J (1989) Regulation of cell adhesion receptors by transforming growth factor-β. Concomitant regulation of integrins that share a common β, subunit. J Biol Chem 264: 380–388
Hemler ME (1990) VLA proteins in the integrin family; structures, functions and their role on leucocytes. Ann Rev Immun 8: 365–400
Howard J, Pilkington GJ (1990) Antibodies to fibronectin bind to plaques and other structures in Alzheimer's disease and control brains. Neurosci Lett 118: 71–76
Hynes RO (1987) Integrins: a family of cell surface receptors. Cell 48: 549–554
Ikeda S-I, Yanagisawa N, Allsop D, Glenner GG (1989) Evidence of amyloid beta-protein immunoreactive early plaque lesions in Down's syndrome brains. Lab Invest 61: 133–137
Ishii T, Haga S (1984) Immuno-electron microscopic localization of complements in amyloid fibrils of senile plaques. Acta Neuropathol (Berl) 63: 269–300
Ignotz RA, Heino J, Massagué J (1989) Regulation of cell adhesion receptors by transforming growth factor-β. Regulation of vitronectin receptor and LFA-1. J Biol Chem 264: 389–392
Joachim CL, Games D, Morris J, Ward P, Frenkel D, Selkoe DJ (1991) Antibodies to non-beta regions of the beta-amyloid precursor protein detect a subset of senile plaques. Am J Pathol 138: 373–384
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grezschik K-H, Multhaup G, Beyreuther K, Müller-Hill B (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell surface receptor. Nature 325: 733–736
Kantor RRS, Mattes MJ, Lloyd KO, Old LJ, Albino AP (1987) Biochemical analysis of two cells surface glycoproteins complexes, very common antigen 1 and very common antigen 2. Relationship to very late activation T cell antigens. J Biol Chem 262: 15158–15165
Keizer GD, Borst J, Frigdor CG, Spits H, Miedema F, Terhorst C, De Vries JE (1985) Biochemical and functional characteristics of the human leucocyte membrane antigen family LFA-1, Mo-1 and p150,95. Eur J Immunol 15: 1142–1147
Keizer GD, Te Velde AA, Schwarting R, Figdor CG, de Vries JE (1987) Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol 17: 1317–1322
Koike F, Kunishita T, Nakayama H, Tabira T (1988) Immunohistochemical study of Alzheimer's disease using antibodies to synthetic amyloid and fibronectin. J Neurol Sci 85: 9–15
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid core protein in Alzheimer's disease and Down's syndrome. Proc Natl Acad Sci (USA) 82: 4245–4249
Masure S, Opdenakker G (1989) Cytokine-mediated proteolysis in tissue remodelling. Experientia 45: 542–549
McGeer PL, Akiyama H, Itagaki S, McGeer EG (1989) Immune system response in Alzheimer's disease. Can J Neurol Sci 16: 516–527
Miedema F, Tetteroo PA, Hesselink WG, Werner G, Spits H, Melief CJ (1984) Both Fc receptors and lymphocyte-function-associated antigen 1 on human T gamma lymphocytes are required for antibody-dependent cellular cytotoxicity (killer cell activity). Eur J Immunol 14: 518–523
Monard D (1988) Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. TINS 11: 541–544
Müller-Hill B, Beyreuther K (1989) Molecular biology of Alzheimer's disease. Annu Rev Biochem 58: 287–307
Murtomaki S, Risteli J, Risteli L, Koivisto U-M, Johansson S, Liesi P (1992) Laminin and its neurite outgrowth promoting domain in the brain in Alzheimer's disease and Down's syndrome. J Neurosci Res 32: 261–273
Narindrasorasak S, Lowery DE, Altman RA, Gonzalez-DeWhitt PA, Greenberg BD, Kisilevsky (1992) Characterization of high affinity binding between laminin and Alzheimer's disease amyloid precursor proteins. Lab Invest 67: 643–652
Pearlstein E, Sorvillo J, Gigli I (1982) The interaction of human plasma fibronectin with a subunit of the first component of complement, C1q. J Immunol 128: 2036–2039
Perlmutter LS, Barrón E, Saperia D, Chui HC (1991) Association between vascular basement membrane components and the lesions of Alzheimer's disease. J Neurosci Res 30: 673–681
Puchtler H, Seat F, Levine H (1962) On the binding of congo red by amyloid. J Histochem Cytochem 10: 355–364
Rogers J, Luber-Narod J, Styzen SD, Civin WH (1988) Expression of immune system-associated antigen by cells of the human central nervous system. Relationship to the pathology of Alzheimer's disease. Neurobiol Aging 9: 330–349
Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P, Lieberburg I (1992). Complement activation by β-amyloid in Alzheimer's disease. Proc Natl Acad Sci (USA) 89: 10016–10021
Rostagno A, Frangione B, Pearlstein E, Garcia-Pardo A (1986) Fibronectin binds to amyloid P component. Localization of the binding site to the 31,000 dalton C-terminal domain. Biochem Biophys Res Commun 140: 12–20
Rozemuller JM, Eikelenboom P, Pals ST, Stam FC (1989a) Microglial cells around amyloid plaques in Alzheimer's disease express leucocyte adhesion molecules of the LFA-1 family. Neurosci Lett 101: 288–292
Rozemuller JM, Eikelenboom P, Stam FC, Beyreuther K, Masters CL (1989b) A4 protein in Alzheimer's disease: primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol 48: 647–663
Rozemuller JM, Abbink JJ, Kamp AM, Stam FC, Hack CE, Eikelenboom P (1991) Distribution pattern and functional state of α1 antichymotrypsin in plaques and vascular amyloid. Acta Neuropathol (Berl) 82: 200–207
Rozemuller JM, Van der Valk P, Eikelenboom P (1992) Activated microglia and cerebral amyloid deposits in Alzheimer's disease. Res Immunol 143: 646–649
Rozemuller JM, Roos RAC, Bots GTAM, Kamphorst W, Eikelenboom P, VanNostrand WE (1993) Distribution of β/A4 protein and amyloid precursor protein in hereditary cerebral hemorrhages with amyloidosis-Dutch type and Alzheimer's disease. Am J Pathol 142: 1449–1457
Ruoslahti E (1991) Integrins. J Clin Invest 91: 1–5
Ruoslahti E, Purschbachter MD (1987) New perspectives in cell adhesion: RGB and integrins. Science 238: 491–497
Sánchez-Madrid F, De Landázuri MO, Morago G, Cebrián M, Acevedo A, Bernabeu C (1986) VLA-3: A novel polypeptide association within the VLA molecules complex: cell distribution and biochemical characterization. Eur J Immunol 16: 1343–1349
Sanes JR (1989) Extracellular matrix molecules that influence neural development. Ann Rev Neurosci 12: 491–516
Schwarting R, Stein H, Wang CY (1985) The monoclonal antibodies αS-HCL1 (αLeu-14) and αS-HCL3 (αLeu M5) allow the diagnosis of hairy cell leukemia. Blood 65: 974–983
Schwartz BR, Carlos TM, Ochs HD, Harlan JM (1990) Identification of surface protein mediating adherence of CD11/CD18 deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest 85: 2019–2022
Selkoe DJ (1991) The molecular pathology of Alzheimer's disease. Neuron 6: 487–498
Selkoe DJ, Abraham CR, Podlisny MB, Buffy LK (1986) Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem 46: 1820–1834
Sjohi M, Hirai S, Yamaguchi H, Harigaya Y, Kawarabayashi T (1990) Amyloid β-protein precursor accumulates in dystrophic neurites of senile plaques in Alzheimer-type dementia. Brain Res 512: 164–168
Sonnenberg A, Modderman PW, Hogervorst F (1988) Laminin receptor on platelets is the integrin VLA-6. Nature 360: 487–489
Springer TA (1990) Adhesion receptors of the immune system. Nature 346: 425–434
Tagliavini F, Giaconne G, Frangione B, Bugiani O (1988) Preamyloid deposits in the cerebral cortex of patients with Alzheimer's disease and nondemented individuals. Neurosci Lett 93: 191–196
Tetteroo PA, Lansdorp PM, Leeksma DC, von dem Borne AE (1983) Monoclonal antibodies against human platelet glycoprotein IIIa. Br J Haematol 55: 509–522
Van der Wal E, Gomez-Pinilla F, Cotman CW (1993) Transforming growth factor-β in plaques in Alzheimer and Down pathologies. Neuroreport 4: 69–72
Yamaguchi H, Hirai S, Morimatsu M, Sjohi M, Ihara Y (1988) A variety of cerebral amyloid deposits in the brains of the Alzheimer type dementia demonstrated by β-protein immunostaining. Acta Neuropathol (Berl) 76: 541–549
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eikelenboom, P., Zhan, S.S., Kamphorst, W. et al. Cellular and substrate adhesion molecules (integrins) and their ligands in cerebral amyloid plaques in Alzheimer's disease. Vichows Archiv A Pathol Anat 424, 421–427 (1994). https://doi.org/10.1007/BF00190565
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00190565