Summary
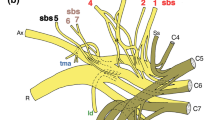
The splenius muscle of the rat was investigated with regard to its structure and innervation. The latter was compared with that of the quadriceps muscle. The results can be summarized as follows: The splenius muscles of both sides form a bipennate muscle plate connecting the occipital bone with the spinous process of the second thoracic vertebra. The lateral parts of both muscles are attached directly to this prominent bony process, whereas the medial parts end in a median raphe which forms a tendinous cranial extension of the second thoracic vertebra. This tendinous extension, showing no connection to the cervical vertebrae, serves also for the attachment of acromio-trapezius muscle fibers. The lateral part of the splenius muscle is divided into two parts by a tendinous intersection.
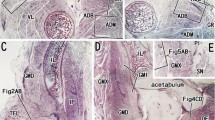
The splenius muscle consists mainly of fast twitch fibers: 55% were characterized as IIB and 40% as IIA fibers by histochemical demonstration of myosin ATPase-activity. A high content of muscle spindles — 57 spindles per gram of muscle tissue — was found.
Comparing several aspects of the innervation of the splenius to that of the quadriceps muscle, the following results could be obtained:
-
1.
The ratio of motor end plate size to muscle fiber volume is significantly higher in the splenius than in the quadriceps muscle.
-
2.
As demonstrated by transganglionary HRP-transport, the main part of labeled splenius afferents to the spinal cord terminates in the central cervical nucleus. Quadriceps afferents, entering the lower thoracic and upper lumbar segments, mainly end in the area of Clarke's column. Several labeled fibers descend to the sixth lumbar and first sacral segments, where they terminate in the area of Stilling's nucleus.
-
3.
A group of primary afferents from both muscles-most probably III- and IV-afferents — projects to the dorsal laminae of the dorsal horn; terminals from the splenius are accumulated in the lateral parts of these laminae, where-us those of the quadriceps are more concentrated in the medial areas.
-
4.
Within the brain stem, most afferents from the splemus terminate in the external cuneate nucleus. Most of the quadriceps afferents course to the gracile nucleus.
-
5.
Terminals from both muscle nerves were found in the area of the spinal vestibular nucleus.
In conclusion, the most conspicuous results were:
-
1)
Besides the segmental projection to the dorsal horn there is an almost exclusive projection of splenius primary afferents to relay nuclei to the cerebellum.
-
2)
The relatively high ratio: end plate size/muscle fiber volume, which is characteristics of finely adjusting muscles.
These results provide additional clues to the understanding of the particular role of the neck muscles in posture and head movement.
Similar content being viewed by others
References
Abrahams VC (1977) The physiology of neck muscles; their role in head movement and maintenance of posture. Can J Physiol Pharmacol 55:332–338
Abrahams VC (1981) Sensory and motor specialization in some muscles of the neck. Trends Neurosci 4:24–27
Abrahams VC, Rose PK, Richmond F (1975) Absence of monosynaptic reflex in dorsal neck muscles of the cat. Brain Res 92:130–131
Abrahams VC, Downey D, Morris O, Richmond FJ (1981) Transganglionic transport of WGA/HRP conjugate in the upper cervical cord of the cat. Abstracts Soc Neuroscience, Los Angeles, 18–23
Ammann B, Gottschall J, Zenker, W (1983) Afferent projections from the rat longus capitis muscle studies by transganglionic transport of HRP. Anat Embryol 166:275–289
Amonoo-Kuofi HS (1982) The number and distribution of muscle spindles in human intrinsic postvertebral muscles. J Anatomy 135:585–599
Anastasopoulos D, Merger T (1982) Canal-neck interaction in vestibular nuclear neurons of the cat. Exp Brain Res 46:269–280
Anzenbacher H, Zenker W (1963) Über die Größenbeziehungen der Muskelfasern zu ihren motorischen Endplatten und Nerven. Z Zellforsch 60:860–871
Bakker GJ, Richmond FJR (1980) Distribution of receptors around neck vertebrae in the cat. J Physiol 298:40–41 p
Bakker GJ, Richmond FJR (1981) Two types of muscle spindles in cat neck muscles: a histochemical study of intrafusal fibre composition. J Neurophysiol 45:973–986
Bakker DA, Richmond FJR (1982) Muscle spindle complexes in muscles around upper cervical vertebrae in the cat. J Neurophysiol 48:62–74
Basbaum AI, Hand PJ (1973) Projections of cervicothoracic dorsal roots to the cuneate nucleus of the rat, with observations on cellular “bricks”. J Comp Neurol 148:347–360
Brodal A (1941) Die Verbindungen des Neucleus cuneatus externus mit dem Kleinhirn beim Kaninchen und bei der Katze. Experimentelle Untersuchungen. Z ges Neurol Psychiat 171:167–199
Brodal A (1975) Neurological Anatomy in relation to clinical medicine. 2nd ed. The Oxford University Press, London, Toronto
Brodal A, Pompeiano O (1957) The vestibular nuclei in the cat. J Anatomy 91:438–454
Cervero F, Jggo A (1980) The substantia gelatinosa of the spinal cord. Brain 103:717–772
Cooke JD, Larsson B, Oscarsson O, Sjödlund B, (1971) Organization of afferent connections to cuneocerebellar tract. Exp Brain Res 13:359–377
Cooper S, Daniel PM (1963) Muscle spindles in man: their morphology in the lumbricales and the deep muscles of the neck. Brain 86:563–586
Cope S, Ryan GMS (1959) Cervical and otolith vertigo. J Laryngol Otol 73:113–120
Corbin KB, Hinsey JC (1935) Intramedullary course of the dorsal root fibers of each of the first four cervical nerves. J Comp Neurol 63:119–126
Ferraro A, Barrera SE (1935) The nuclei of the posterior funiculi in Macacus rhesus. An anatomical and experimental investigation. Arch Neurol Psychiat (Chicago) 33:262–275
Gottschall J, Neuhuber W, Müntener M, Mysicka A (1980a) The ansa cervicalis and the infrahyoid muscles of the rat. II. Motor and sensory neurons. Anat Embryol 159:59–69
Gottschall J, Zenker W, Neuhuber W, Mysicka A, Müntener M (1980b) The sternomastoid muscle of the rat and its innervation. Muscle fiber composition, perikarya and axons of efferent and afferent neurons. Anat Embryol 160:285–300
Graham, RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14:291–302
Grant G, Ygge J (1981) Somatotopic organization of the thoracic spinal nerve in the dorsal horn demonstrated with transganglionic degeneration. J Comp Neurol 202:357–364
Gruber H (1966) Die Größenbeziehung von Muskelfaservolumen und Fläche der motorischen Endplatte bei verschiedenen Skelettmuskeln der Ratte. Acta Anat 64:628–633
Guth L, Samaha FJ (1970) Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol 28:365–367
Heckmann T, Bourassa CM (1981) Lesions of the dorsal column nuclei or medial lemniscus of the cat: effect on motor performance. Brain Res. 224:405–411
Hirai N, Hongo T, Sasaki S (1978) Cerebellar projection and input organizations of the spinocerebellar tract arising from the central cervical nucleus of the cat. Brain Res. 157:341–345
Johnson JI Jr, Welker WI, Pubols BH Jr (1968) Somatotopic organization of racoon dorsal column nuclei. J Comp Neurol 132:1–44
Jongkees LB (1969) Cervical vertigo. Laryngoscope 79:1473–1484
Katan S, Gottschall, J, Neuhuber W (1982) Simultaneous visualization of horseradish peroxidase and nuclear yellow in tissue sections for neuronal double labeling. Neurosci Lett 28:121–126
Kerr FWL (1961) Structural relation of the trigeminal spinal tract to upper cervical roots and the solitary nucleus of the cat. Ex Neurol 4:134–148
Koelle GB, Friedenwald JS (1949) A histochemical method for localizing cholinesterase activity. Proc Soc Exp Biol Med (NY) 70:617–622
Landgren S, Silfvenius H (1969) Projection to cerebral cortex of group I muscle afferents from the cat's hindlimb. J Physiol 200:353–372
Langford LA, Goggeshall RE (1981) Branching of sensory axons in the peripheral nerve of the rat. J Comp Neurol 203:745–750
Matsushita M, Ikeda M (1975) The central cervical nucleus as cell origin of a spinocerebellar tract arising from the cervical cord: a study in the cat using horseradish peroxidase. Brain Res 100:412–419
Matsushita M, Hosoya Y (1979) Cells of origin of the spinocerebellar tract in the rat, studied with the method of retrograde transport of horseradish peroxidase. Brain Res 173:185–200
McCouch GP, Deering ID, Ling TH (1951) Location of receptor for tonic neck reflexes. J Neurophysiol 14:191–195
Merger T, Anastasopoulos D, Becker W (1982): Neuronal responses to horizontal neck deflection in the group X region of the cat's medullary brain stem. Exp Brain Res 45:196–206
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Müntener M (1979) Variable pH dependence of the myosin-ATPase in different muscles of the rat. Histochemistry 62:299–304
Müntener M (1982) A rapid and reversible muscle fiber transformation in the rat. Exp Neurol 77:668–678
Müntener M, Gottschall J, Neuhuber W, Mysicka A (1980) The ansa cervicalis and the infrahyoid muscles of the rat. I. Anatomy; distribution, number and diameter of fiber types; motor units. Anat Embryol 159:49–57
Mysicka A, Zenker W (1981) Central projections of muscle afferents from the sternomastoid nerve in the rat. Brain Res 211:257–265
Neuhuber W, Niederle B, Zenker W (1977) Somatopetal transport of horseradish peroxidase (HRP) in the peripheral and central branches of dorsal root ganglion cells. Cell Tissue Res 183:395–402
Ogata T, Hondo T, Seito T (1967) An electron microscopic study on differences in the fine structures of motor end plate in red, white and intermediate muscle fibers of rat intercostal muscle. A preliminary study. Acta Med Okayama 21:327–338
Oscarsson O (1965) Functional organization of the spino- and cuneocerebellar tracts. Physiol Rev 45:495–522
Padykula H, Gauthier G (1970) The ultrastructure of the neuromuscular junctions of mammalian red, white and intermediate skeletal muscle fibers. J Cell Biol 46:27–41
Pellegrino LJ, Pellegrino AS, Cushman AJ (1979) Stereotaxic atlas of the rat brain. Plenum Press, New York
Petras JM, Cummings JF (1975) Cervical spinocerebellar projections in the dog. Anat Rec 181:448
Raberger E (1972) Innervationsunterschiede zwischen roten und weißen Muskelfasern der Ratte. Anat. Anz 130: Erg H, 431–434
Ranson SW, Davenport HK, Doles EA (1932) Intramedullary course of the dorsal root fibers of the first three cervical nerves. J Comp Neurol 54:1–12
Rapoport S (1979) Reflex connexions of motoneurones of muscles involved in head movement in the cat. J Physiol 289:311–327
Richmond FJR, Abrahams VC (1975a) Morphology and enzyme histochemistry of dorsal muscles of the cat neck. J Neurophysiol 38:1312–1321
Richmond FJR, Abrahams VC (1975b) Morphology and distribution of muscle spindles in dorsal muscles of the cat neck. J Neurophysiol 38:1322–1339
Richmond FJR, Abrahams VC (1979) Physiological properties of muscle spindles in dorsal neck muscles of the cat. J Neurophysiol 42:604–617
Richmond FJR, Anstee GCB, Sherwin EA, Abrahams VC (1976) Motor and sensory fibres of neck muscle nerves in the cat. Can J Physiol Pharmacol 54:294–304
Richmond FJR, Bakker DA (1982) Anatomical organization and sensory receptor content of soft tissues surrounding upper cervical vertebrae in the cat. J Neurophysiol 48:49–61
Rose PK, Twiddy DAS (1981) The organization of stem dendrites of dorsal neck muscle motoneurons in the adult cat. Abstracts. Soc Neurosciences, Los Angeles, 18–23
Rosen I (1969) Localization in caudal brain stem and cervical spinal cord of neurons activated from forelimb group I afferents in the cat. Brain Res 16:55–71
Scheurer S, Gottschall J, Groh V (1983) Afferent projections of the rat major occipital nerve studied by transganglionic transport of HRP. (in press)
Snyder RL, Faull RLM, Mehler WR (1978) A comparative study of the neurons of origin of the spinocerebellar afferents in the rat, cat and squirrel monkey, based on the retrograde transport of horseradish peroxidase. J Comp Neurol 181:833–852
Stillhard G (1981) Musculi longus capitis et splenius der Ratte und innervierende Motoneurone. Acta Neuropathol 53:267–274
Voss H (1958) Zahl und Anordnung der Muskelspideln in den unteren Zungenbeinmuskeln, dem M. sternocleidomastoideus und den Bauch- und tiefen Nackenmuskeln. Anat Anz 105:265–275
Wiksten B (1975) The central cervical nucleus — A source of spinocerebellar fibers, demonstrated by retrograde transport of horseradish peroxidase. Neurosci Lett 1:81–84
Wiksten B (1979a) The central cervical nucleus in the cat. I. A Golgi study. Exp Brain Res 36:143–154
Wiksten B (1979b) The central cervical nucleus in the cat. III. The cerebellar connexions studied with anterograde transport of 3H-leucine. Exp Brain Res 36:175–189
Wilson VJ, Maeda M, Frank JI, Shimazu H (1976) Mossy fiber neck and second-order labyrinthine projections to cat flocculus. J Neurophysiol 39:301–310
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pfister, J., Zenker, W. The splenius capitis muscle of the rat, architecture and histochemistry, afferent and efferent innervation as compared with that of the quadriceps muscle. Anat Embryol 169, 79–89 (1984). https://doi.org/10.1007/BF00300589
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00300589