Summary
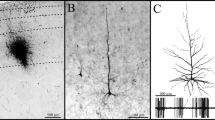
Callosally projecting neurons, labeled following injection of horseradish peroxidase (HRP) into the 17/18a border of the contralateral hemisphere, have been examined by light and electron microscopy. These neurons exhibit two types of horseradish peroxidase labeling: either a diffuse, Golgi-like labeling, or a granular, punctate labeling. The punctate type of HRP-labeling is the predominant form in nonpyramidal neurons, while pyramidal neurons frequently display either diffuse or punctate labeling. Only punctately labeled neurons have been examined in this study. Light microscopic analyses of 1-μm sections show that in the heavily labeled zone at the area 17/18a border approximately 9% of all of the cells in layer II/III are callosally projecting nonpyramidal cells, and 70% of them are callosally projecting pyramidal cells. Light and electron microscopic examinations indicate that the nonpyramidal neurons are a heterogeneous group which consists of small multipolar neurons, large multipolar neurons, small bipolar neurons, and large bipolar neurons. To investigate the ultrastructural appearance of the punctate HRP labeling, selected neurons have been examined in thin sections. In the electron microscope, the tetramethylbenzidine (TMB) reaction product appears as electron-dense crystals, while the diaminobenzidine (DAB) reaction product appears as dark, electron-dense material which fills the lysosomes. These lysosomes occasionally have a halo of reaction product, but often they are not morphologically distinguishable from dark lysosomes present within neurons from control animals in which the darkening results from staining the thin sections with lead citrate and uranyl acetate. However, labeled neurons possess more dark lysosomes than neurons from control animals. These additional dark lysosomes presumably contain the HRP reaction product visible by light microscopy.
Similar content being viewed by others
References
Adams JC (1981) Heavy metal intensification of DAB — based HRP reaction product. J Histochem Cytochem 29:775
Bakay RAE, Harris AB (1981) Neurotransmitter, receptor and biochemical changes in monkey cortical epileptic foci. Brain Res 206:387–404
Benes FM (1990) Alterations in corticolimbic circuitry may be linked to schizophrenia. Psychiatr Times VII(4):12–13
Benes FM, McSparren J, Sangiovanni JP, Vincent SL (1989) Deficits in small interneurons in schizophrenic cortex. Soc Neurosci Abstr 15:449.6
Berlucchi G (1972) Anatomical and physiological aspects of visual functions of corpus callosum. Brain Res 37:371–392
Bishop PO, Henry GH (1971) Spatial vision. Ann Rev Psych 22:119–160
Bleuler E (1950) Dementia Praecox. International Universities Press, New York
Broadwell RD, Brightman MW (1979) Cytochemistry of undamaged neurons transporting exogenous protein in vivo. J Comp Neurol 185:31–74
Buhl EH, Singer W (1989) The callosal projection in cat visual cortex as revealed by a combination of retrograde tracing and intracellular injection. Exp Brain Res 75:470–476
Code RA, Winer JA (1985) Commissural neurons in layer III of cat primary auditory cortex (AI): Pyramidal and non-pyramidal cell input. J Comp Neurol 242:485–510
Cook ND (1984) Homotopic callosal inhibition. Brain Lang 23:116–125
Diao Y, Wang YK, Pu ML (1983) Binocular responses of cortical cells and the callosal projection in the albino rat. Exp Brain Res 49:410–418
Emson PC (1985) Neurotransmitter systems. In: Bousfield D (ed) Neurotransmitters in action. Elsevier Biomedical Press, Amsterdam New York Oxford, pp 6–10
Espinoza SG, Thomas HC (1983) Retinotopic organization of striate and extrastriate visual cortex in hooded rat. Brain Res 272:137–144
Feldman ML, Peters A (1978) The forms of nonpyramidal neurons in the visual cortex of the rat. J Comp Neurol 179:761–794
Gardner JC, Cynader MS (1987) Mechanisms for binocular depth sensitivity along the vertical meridian of the visual field. Brain Res 413:60–74
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: Ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14(4):291–302
Hallman LE, Schofield BR, Lin C (1988) Dendritic morphology and axon collaterals of corticotectal, corticopontine, and callosal neurons in layer V of primary visual cortex of the hooded rat. J Comp Neurol 272:149–160
Hornung JP, Garey LJ (1981) Ultrastructure of visual callosal neurons in cat identified by retrograde axonal transport of horseradish peroxidase. J Neurocytol 10:297–314
Hughes CM, Peters A (1990) Morphological evidence for callosally projecting nonpyramidal neurons in rat visual cortex. J Anat Embryol 182(6):591–603
Hughes CM, Peters A (1992) Symmetric synapses formed by callosal afferents in rat visual cortex. Brain Res (in press)
Innocenti GM (1980) The primary visual pathway through the corpus callosum: morphological and functional aspects in the cat. Arch Italian Biol 118:124–188
Kosaka T, Heizmann CW, Tateishi K, Hamaoka Y, Hama K (1987) An aspect of the organizational principle of the gamma-aminobutyric acid-ergic system in the cerebral cortex. Brain Res 409:403–408
Kraepelin E (1971) Dementia praecox. Krieger, New York
LeVay S (1973) Synaptic patterns in the visual cortex of the cat and monkey. Electron microscopy of Golgi preparations. J Comp Neurol 150:53–86
Lund JS (1984) Spiny stellate neurons. In: Peters A, Jones EG (eds) Cerebral cortex, vol 1, Plenum Press, New York, pp 255–308
Marfurt CF, Turner DF, Adams CE (1988) Stabilization of tetramethylbenzidine (TMB) reaction product at the electron microscopic level by ammonium molybdate. J Neurosci Methods 25:215–223
Meinecke DL, Peters A (1986) Somatostatin immunoreactive neurons in rat visual cortex: a light and electron microscopic study. J Neurocytol 15:121–136
Meinecke DL, Peters A (1987) GABA immunoreactive neurons in rat visual cortex. J Comp Neurol 261:388–404
Miller M, Vogt B (1984) Heterotopic and homotopic callosal connections in rat visual cortex. Brain Res 294:75–89
Mitchell DE, Blakemore C (1970) Binocular depth perception and the corpus callosum. Vision Res 10:49–54
Olavarria J, Van Sluyters RC (1983) Widespread callosal connections in infragranular visual cortex of the rat. Brain Res 279:233–237
Parnavelas JG, Lieberman AR, Webster KE (1977a) Organization of neurons in the visual cortex, area 17, of the rat. J Anat 124:305–322
Parnavelas JG, Sullivan K, Lieberman AR, Webster KE (1977b) Neurons and their synaptic organization in the visual cortex of the rat: Electron microscopy of Golgi preparations. Cell Tissue Res 183:499–517
Parnavelas JG, Dinopoulos A, Davies SW (1989) The central visual pathways. In: Bjorklund A, Hokfelt T, Swanson LW (eds) Handbook of chemical neuroanatomy, vol 7. Elsevier, Amsterdam New York Oxford, pp 1–164
Peters A, Fairén A (1978) Smooth and sparsely-spined stellate cells in the visual cortex of the rat: a study using a combined Golgi-electron microscopic technique. J Comp Neurol 181:129–172
Peters A, Harriman KM (1988) Enigmatic bipolar cell of rat visual cortex. J Comp Neurol 267:409–432
Peters A, Kara D (1985a) The neuronal composition of area 17 of rat visual cortex. I. The pyramidal cells. J Comp Neurol 234:218–241
Peters A, Kara D (1985b) The neuronal composition of area 17 of rat visual cortex. II. The nonpyramidal cells. J Comp Neurol 234:242–263
Peters A, Kimerer LM (1981) Bipolar neurons in rat visual cortex: a combined Golgi-electron microscopic study. J Neurocytol 10:921–946
Peters A, Proskauer CC (1980) Smooth or sparsely spined cells with myelinated axons in rat visual cortex. Neurosci 5:2079–2092
Peters A, Proskauer CC, Ribak CE (1982) Chandelier cells in rat visual cortex. J Comp Neurol 206:397–416
Peters A, Meinecke DL, Karamanlidis AN (1987) Vasoactive intestinal polypeptide immunoreactive neurons in the primary visual cortex of the cat. J Neurocytol 16:23–38
Peters A, Payne BR, Josephson K (1990) Transcallosal non-pyramidal cell projections from visual cortex in the cat. J Comp Neurol 32:124–142
Ribak CE (1978) Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J Neurocytol 7:461–478
Ribak CE (1985) Axon terminals of GABAergic chandelier cells are lost at epileptic foci. Brain Res 326:251–260
Ribak CE, Harris AB, Vaughn JE, Roberts E (1979) Inhibitory, GABAergic nerve terminals decrease at sites of focal epilepsy. Science 205:211–214
Ribak CE, Hunt CA, Bakay RAI, Oertel WH (1986) A decrease in the number of GABAergic somata is associated with the preferential loss of GABAergic terminals at epileptic foci. Brain Res 363:78–90
Somogyi P (1977) A specific axo-axonal interneurons in the visual cortex of the rat. Brain Res 136:345–350
Swadlow HA (1974) Properties of antidromically activated callosal neurons and neurons responsive to callosal input in rabbit binocular cortex. Exp Neurol 43:424–444
Thomas HC, Espinoza SG (1987) Relationships between interhemispheric cortical connections and visual areas in hooded rats. Brain Res 417:214–224
Turner PT, Harris AB (1974) Ultrastructure of exogenous peroxidase in cerebral cortex. Brain Res 74:305–326
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hughes, C.M., Peters, A. Types of callosally projecting nonpyramidal neurons in rat visual cortex identified by lysosomal HRP retrograde labeling. Anat Embryol 186, 183–193 (1992). https://doi.org/10.1007/BF00174956
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00174956