Abstract
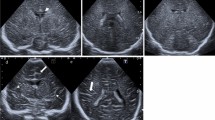
We present a 2-year-old boy and a 6-year-old girl with mild Canavan disease (CD). Aspartoacylase activity in skin fibroblasts was deficient. Magnetic resonance imaging (MRI) of the brain did not show the prominent leucodystrophy previously reported in CD, but there was a hyperintense signal from the lentiform nuclei and the heads of the caudate nuclei on the T2-weighted MR images. This suggests a specific vulnerability of the corpus striatum in these patients. In the older patient, the white matter became affected at the age of 6 years. Proton magnetic resonance spectroscopy (1H-MRS) of white matter revealed a normal concentration of N-acetyl-l-aspartate (NAA) and a markedly decreased concentration of choline containing compounds (Cho) in the boy but a normal ratio of NAA to Cho in the girl. We conclude that deficient NAA catabolism affects myelin metabolism. This may present as changes in the striatum and/or as a low concentration of Cho before leucodystrophy appears on MRI.
Similar content being viewed by others
Abbreviations
- CD:
-
Canavan disease
- Cho:
-
choline containing compounds
- 1H-MRS:
-
1H-magnetic resonance spectroscopy
- MRI:
-
magnetic resonance imaging
- NAA:
-
N-acetyl-l-aspartate
- VOI:
-
volume of interest
References
Austin SJ, Connelly A, Gadian DG, Benton JS, Brett EM (1991) Localized 1H NMR spectroscopy in Canavan's disease: a report of two cases. Magn Reson Med 19:439–445
Bottomley PA (1987) Spatial localization in NMR spectroscopy in vivo. NY Acad Sci 508:333–348
Christensen E, Jacobsen BB, Gregersen N, Hjeds H, Pedersen JB, Brandt NJ, Bækmark UB (1981) Urinary excretion of succinylacetone and delta-aminolevulinic acid in patients with hereditary tyrosinemia. Clin Chim Acta 116:331–341
Christiansen P, Henriksen O, Stubgaard M, Gideon P, Larsson HBW (1983) In vivo quantification of brain metabolites by 1H-MRS using water as an internal standard. Magn Reson Imaging 11:107–118
Divry P, Vianey-Liaud C, Gay C, Macabeo V, Rapin F, Echenne B (1988) N-acetylaspartic aciduria: report of three new cases in children with a neurological syndrome associating macrocephaly and leukodystrophy. J Inherited Metab Dis 11: 307–308
Edwards MK, Bonin JM (1991) White matter diseases. In: Atlas SW (ed) Magnetic resonance imaging of the brain and spine, 2nd edn. Raven Press, New York, pp 486–487
Frahm J, Michaelis T, Merboldt KD, Bruhn H, Gyngell ML, Hänicke W (1990) Improvements in localized proton NMR spectroscopy of human brain. Water suppression, short echo times, and 1 ml resolution. Magn Reson Med 90:464–473
Grodd W, Krägeloh-Mann I, Klose U, Sauter R (1991) Metabolic and destructive brain disorders in children: findings with localized proton MR spectroscopy. Radiology 181:173–181
Hagenfeldt L, Bollgren I, Venizelos N (1987) N-acetylaspartic aciduria due to aspartoacylase deficiency — a new aetiology of childhood leukodystrophy. J Inherited Metab Dis 10:135–141
Knaap MS van der, Grond J van der, Rijen PC van, Faber JA, Valk J, Willemse K (1990) Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology 176:509–515
Kvittingen EA, Guldal G, Borsting S, Skalpe IO, Stokke O, Jellum E (1986) N-acetylaspartic aciduria in a child with a progressive cerebral atrophy. Clin Chim Acta 158:217–227
Marks HG, Caro PA, Wang ZY, Detre JA, Bogdan AR, Gusnard DA, Zimmerman RA (1991) Use of computed tomography, magnetic resonance imaging, and localized 1H magnetic resonance spectroscopy in Canavan's disease: a case report. Ann Neurol 30:106–110
Matalon R, Michals K, Sebesta D, Deanching M, Gashkoff P, Casanova J (1988) Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am J Med Genet 29:463–471
Matalon R, Kaul R, Casanova J, Michaels K, Johnson A, Rapin I, Gashkoff P, Deanching M (1989) SSIEM Award. Aspartoacylase deficiency: the enzyme defect in Canavan disease. J Inherited Metab Dis 12 [Suppl 2]:329–331
Miller BL (1991) A review of chemical issue in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 4:47–52
Norton WT, Poduslo SE, Suzuki K (1966) Suacute sclerosing leukoencephalitis. II. Chemical studies including abnormal myelin and an abnormal ganglioside pattern. J Neuropathol Exp Neurol 25:582–597
Valk J, Knaap MS van der (1989) Magnetic resonance of myelin, myelination and myelin disorders. Springer, Berlin Heidelberg New York, pp 137–139
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toft, P.B., Geiß-Holtorff, R., Rolland, M.O. et al. Magnetic resonance imaging in juvenile Canavan disease. Eur J Pediatr 152, 750–753 (1993). https://doi.org/10.1007/BF01953994
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01953994