Summary
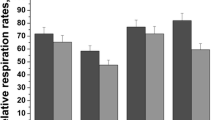
Two-thirds hepatectomy in rats resulted in elevated blood ethanol and acetaldehyde levels as compared to those of sham operated and CCl4-induced toxic injured rats. The acetaldehyde/ethanol ratio increased also. Although the liver mass regenerated within 3 days, ethanol metabolism remained disturbed. Mitochondrial aldehyde dehydrogenase activity was significantly diminished only following partial hepatectomy. The results suggest that abnormal ethanol and especially acetaldehyde metabolism in partially hepatectomized rats is not due simply to reduced liver tissue but to a diminished aldehyde dehydrogenase activity in the remaining tissue.
Similar content being viewed by others
References
Buttner H (1965) Aldehyd- und Alkoholdehydrogenase Aktivität in Leber und Niere der Ratte. Biochem Z 341:300–314
Ebina Y, Iwai H, Fukui N, Ohtsuka H, Miura Y (1975) Prereplicative enzymatic changes in regenerating rat liver. J Biol Chem 77:641–645
Hasumura Y, Teschki R, Lieber CS (1975) Acetaldehyde oxidation by hepatic mitochondria: decrease after chronic ethanol consumption. Science 189:727–728
Higgins GM, Anderson RM (1931) Experimental pathology of the liver. 1. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12:186–202
Imai Y, Sato R (1966) Activation and inhibition of microsomal hydroxylation by ethyl isocyanide. Biochem Biophys Res Commun 25:80–86
Korsten MA, Matsuzaki S, Feinman L, Lieber CS (1975) High blood acetaldehyde levels after ethanol administration. Difference between alcoholic and nonalcoholic subjects. N Engl J Med 292:386–389
Lieber CS, DeCarli LM (1968) Ethanol oxidation by hepatic microsomes. Adaptive increase after ethanol feeding. Science 162:917–918
Lieber CS (1980) Alcohol, protein metabolism, and liver injury. Gastroenterology 79:373–376
Lieberman FL (1963) The effect of liver disease on the role of ethanol metabolism in man. Gastroenterology 44:261–266
Lindros KO (1957) Regulatory factors in hepatic acetaldehyde metabolism during ethanol oxidation. In: Lindros KO, Eriksson A (eds) The role of the acetaldehyde in actions of ethanol. Finnish Foundation of Alcohol Studies, Helsinki, pp 67–81
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Okada T, Mizoi Y (1982) Studies on the problem of blood acetaldehyde determination in man and its level after alcohol intake. Jp J Alcohol Drug Dependence 17:141–159
Omura T, Sato R (1964) The carbon monoxide binding pigment of liver microsomes. 1. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378
Palmer KR, Jenkins WJ (1982) Impaired acetaldehyde oxidation. Gut 23:729–733
Schmidt G, Thanhauser SJ (1945) A method for the determination of deoxyribonucleic acid, ribonucleic acid, and phosphoproteins in animal tissues. J Biol Chem 161:83–89
Taketa K, Tanaka A, Watanabe A, Takesue A, Aoe H, Kosaka K (1976) Undifferentiated patterns of key carbohydrate-metabolizing enzymes in injured livers. Enzyme 21:158–173
Tottmar SOL, Pettersson H, Kiessling K-H (1973) The subcellular distribution and properties of aldehyde dehydrogenase in rat liver. Biochem J 135:577–586
Wands JR, Carter EA, Bucher NLR, Isselbacher KJ (1979) Inhibition of hepatic regeneration in rats by acute and chronic ethanol intoxication. Gastroenterology 77:528–531
Watanabe A, Miyazaki M, Taketa K (1976) Differential mechanisms of increased alpha-fetoprotein production in rats following carbon tetrachloride injury and partial hepatectomy. Cancer Res 36:2171–2175
Watanabe A, Kosaka K (1980) Differential mechanisms of increased alpha-fetoprotein production in rats following carbon tetrachloride injury and partial hepatectomy. In: Hirai H, Tsukada Y (eds) Oncofetal proteins. Nankodo, Tokyo, pp 118–123
Watanabe A, Higashi T, Hayashi S, Nagashima H (1982) Insulin and glucagon concentrations in the portal and glucagon concentrations in liver-injured and partially hepatectomized rats. Gastroenterol Jpn 17:36–41
Watanabe A, Shiota T, Hayashi S, Sakata T, Nagashima H (1984) Pleomorphism of hepatic regeneration. Res Exp Med 184:49–57
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watanabe, A., Hobara, N., Nakatsukasa, H. et al. Impaired acetaldehyde metabolism in partially hepatectomized rats. Res. Exp. Med. 185, 13–20 (1985). https://doi.org/10.1007/BF01851523
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01851523