Abstract
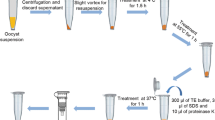
As an alternative to morphological speciation, DNA probes have been developed to identifyEimeria crandallis andE. ovinoidalis, the species associated with ovine coccidiosis. Coccidia-free lambs were infected with pure cultures ofE. crandallis andE. ovinoidalis. Oocysts separated from faeces were sporulated and treated to release sporozoites from which DNA was purified. Repetitive DNA sequences were identified by hybridisation and cloned. The specificity of selected clones was tested by hybridisation with dot blots of four other ovineEimeria species, a mixture of caprineEimeria species andE. acervulina of the chicken. The probes allowed the detection of DNA equivalent to that present in approximately 1,500 oocysts. To increase the sensitivity of the test, a polymerase chain reaction (PCR) approach was adopted, using oligonucleotide primers specific for the cloned repeats. By this method, less than 10 oocysts ofE. ovinoidalis could be reliably detected. In addition to the species-specific probes, other highly repetitive DNA sequences fromE. crandallis andE. ovinoidalis were cloned and sequenced; these repeats hybridised to all ovine, caprine and avianEimeria species tested and contained tandem CGA and putative telomeric repeats.
Similar content being viewed by others
References
Aslud L, Franzen L, Westin G, Persson T, Wigzell H, Petterson U (1985) Highly reiterated non-coding sequence in the genome ofPlasmodium falciparum is composed of 21 base-pair repeats. J Mol Biol 185:509–516
Barker DC (1987) DNA diagnosis of human leishmaniasis. Parasitol Today 3:177–184
Blackburn EH, Szostak JW (1984) The molecular structure of centromeres and telomeres. Annu Rev Biochem 53:163–194
Catchpole J, Norton CC, Joyner LP (1975) The occurrence ofEimeria weybridgensis and other species of coccidia in lambs in England and Wales. Br Vet J 131:392–401
Catchpole J, Norton CC, Joyner LP (1976) Experiments with defined multispecific coccidial infections in lambs. Parasitology 72:137–147
Clarke LE, Messer LI, Greenwood NM, Wisher MH (1987) Isolation of amp3 genomic recombinants coding for antigens ofEimeria tenella. Mol Biochem Parasitol 22:79–85
Dulski P, Turner M (1988) The purification of sporocysts and sporozoites fromEimeria tenella oocysts using Percoll density gradients. Avian Dis 32:235–239
Feinberg AP, Vogelstein B (1983) A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Franzen L, Westin G, Shabo R, Aslud L, Perlmann H, Persson T, Wigzell H, Petterson U (1984) Analysis of clinical specimens by hybridisation with probe containing repetitive DNA fromPlasmodium falciparum. Lancet: 525–528
Gibson WC, Dukes P, Gashumba JK (1988) Species-specific DNA probes for the identification of African trypanosomes in tsetse flies. Parasitology 97:63–73
Gregory MW, Joyner LP, Catchpole J, Norton CC (1980) Ovine coccidiosis in England and Wales. Vet Rec 106:461–462
Hamada H, Petrino MG, Kakunaga T (1982) A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Nat Acad Sci USA 79:6465–6469
Jackson ARB (1962) Excystation ofEimeria arloingi (Marotel, 1905): Stimuli from the host sheep. Nature 194:847–849
Joyner LP, Norton CC, Davies SFM, Watkins CV (1966) The species of coccidia occurring in cattle and sheep in the South-West of England. Parasitology 56:531–541
Ko C, Smith CK, McDonell M (1990) Identification and characterization of a target antigen of a monoclonal antibody directed againstEimeria tenella merozoites. Mol Biochem Parasitol 41:53–64
Liberator PA, Hsu J, Turner MJ (1989) Tandem trinucleotide repeats throughout the nucleotide sequence of a cDNA encoding anEimeria tenella sporozoite antigen. Nucleic Acids Res 17:7104
Macedo AM, Melo MN, Gomes RF, Pena SDJ (1992) DNA fingerpritns: a tool for identification and determination of the relationships between species and strains ofLeishmania. Mol Biochem Parasitol 53:63–70
Majiwa PAO, Masake RE, Nantulya VM, Hamers R, Matthyffenf G (1985)Trypanosoma (Nannomonas) congolense-identification of two karyotypic groups. EMBO J 4:3307–3313
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Ponzi M, Pace T, Dore E, Frontali C (1985) Identification of a telomeric DNA sequence inP. berghei. EMBO J 4:2291–2295
Pout DD (1965) Coccidiosis in lambs. Vet Rec 77:887–888
Pout DD, Ostler DC, Joyner LP, Norton CC (1966) The coccidial population in clinically normal sheep. Vet Rec 78:455–460
Rogers J (1983) CACA sequences-the ends and the means? Nature 305:101–102
Saiki RH, Scharf S, Faloona F, Mullis K, Horn GT, Erlich HA, Arnheim N (1985) Enzymatic amplification of B-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 203:1350–1354
Sanger F, Nicklen S, Coulson AR (19777) DNA sequencing with chain-terminating inhibitors. Proc Nat Acad Sci USA 74:5463–5467
Shirley MW (1993) Molecular karyotypes ofEimeria tenella. In: Barta TR, Fernando MA (eds) Proceedings of the VIth International Coccidiosis Conference, June 21–29, 1993, Guelph, Ontario, Canada. Ontario Veterinary College, Department of Pathology, Guelph, p 144
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–507
Stucki U, Braun R, Roditi I (1993)Eimeria tenella: characterization of a 5S ribosomal RNA repeat unit and its use as a species-specific probe. Exp Parasitol 76:68–75
Van Belkum A, Ramesar J, Trommelen G, Uitterlinden AG (1992) Mini- and microsatellites in the genome of rodent malaria parasites. Gene 118:81–86
Van der Ploeg LTH, Bernards A, Rijsewijk FAM, Borst P (1982) Characterization of the DNA duplication-transposition that controls the expression of two genes for variant surface glycoproteins inTrypanosoma brucei. Nucleic Acids Res 10:593–609
Walmsley RM, Szostak JW, Petes JW (1983) Is there left-handed DNA at the ends of yeast chromosomes? Nature 302:84–86
Wirth DF, McMahon-Pratt D (1982) Rapid identification ofLeishmania species by specific hybridisation of kinetoplast DNA in cutaneous lesions. Proc Natl Acad Sci USA 79:6999–7003
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berriatua, E., Gibson, W.C. & Morgan, K.L. Development of DNA probes for the ovine Eimeria speciesE. crandallis andE. ovinoidalis . Parasitol Res 81, 222–229 (1995). https://doi.org/10.1007/BF00937113
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00937113