Abstract
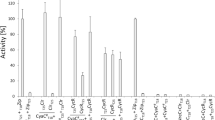
The cellular cAMP level is markedly down-regulated by cAMP receptor protein (CRP) in Escherichia coli. CRP regulates adenylate cyclase both at the level of transcription of its structural gene cya and at the level of enzyme activity. We established a method to determine the phosphorylation state of IIAGlc, the glucose-specific phosphotransferase protein, in intact cells. We found that IIAGlc exists predominantly in the unphosphorylated form in wild-type cells growing in LB medium, while it is largely phosphorylated in crp or cya cells. Disruption of the ptsG gene that codes for the membrane component of the major glucose transporter (IICBGlc), and/or the fruF gene coding for FPr (fructose-specific hybrid phosphotransferase protein), did not affect the phosphorylation state of IIAGlc. When IICBGlc was overproduced in the presence of glucose, the levels of both cAMP and phosphorylated IIAGlc in crp cells were concomitantly decreased to wild-type levels. In addition, when His-90 in IIAGlc was replaced by glutamine, both phosphorylation of IIAGlc and the overproduction of cAMP in crp cells were eliminated. We also found that extracts of crp + cells markedly stimulate dephosphorylation of IIAGlc-P in vitro. We conclude that CRP-cAMP down-regulates adenylate cyclase primarily by reducing the level of phosphorylated IIAGlc. The data suggest that unspecified proteins whose expression is under the control of CRP-cAMP are responsible for this regulation.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 19 March 1998 / Accepted: 18 May 1998
Rights and permissions
About this article
Cite this article
Takahashi, H., Inada, T., Postma, P. et al. CRP down-regulates adenylate cyclase activity by reducing the level of phosphorylated IIAGlc, the glucose-specific phosphotransferase protein, in Escherichia coli . Mol Gen Genet 259, 317–326 (1998). https://doi.org/10.1007/s004380050818
Issue Date:
DOI: https://doi.org/10.1007/s004380050818