Summary
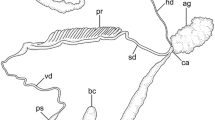
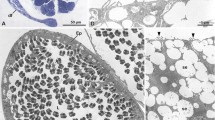
The prosobranch Fusitriton oregonensis exhibits an unusual form of sperm polymorphism. The viable, eupyrene sperm are attached in groups of about fifty to worm-shaped, apyrene, carrier sperm. There is a second apyrene sperm, which is lancet-shaped and has a different internal organization than the carrier, but does not transport eupyrene sperm.
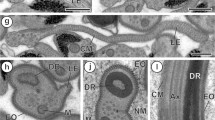
The eupyrene sperm are filiform (185 μm long), with a conical acrosome, elongate nucleus and midpiece. They contain large stores of glycogen in the principal piece, together with an unusually high proportion of protein. The latter is due to a complex interconnecting system of fibres that supports the tail internally. A distinct annulus is located, characteristically, at the junction between midpiece and principal piece.
The carrier sperm has a core of about 112 axonemes that arise from basal bodies in the anterior end and extend through its entire length of 36 μm. The basal bodies have unstriated rootlets that are embedded in a granular cap. Large membrane-bound “yolk bodies” are arranged along the length of the carrier sperm, on either side of the median axonemal core. Dense bodies, which may be indigestible residues formed from the degeneration of the nucleus, are excreted by exocytosis. Individual carrier sperm are capable of “corkscrew” propulsion, resembling that of spirochaetes.
The lancet sperm is three times as long as the carrier. The sixteen or so axonemes, which are arranged peripherally like a cage enclosing the cytoplasm, originate from a dense centriolar plate in the anterior end. The cytoplasm is filled with secretions including small yolk granules, dense bodies (also excreted), clear vesicles, and a membranated granular secretion that resembles mucus. The possible functions of the lancet and carrier sperm are discussed.
Similar content being viewed by others
References
Anderson WA, Personne P (1976) The molluscan spermatozoon: Dynamic aspects of its structure and function. Am Zool 16:293–313
Anderson WA, Personne P, André J (1968) Chemical compartmentalization in Helix spermatozoa. J Microsc 7:367–390
Ankel WE (1924) Spermatozoendimorphismus und Befruchtung bei Bithynia tentaculata L. und Viviparus viviparus L. Senckenbergiana 6:1–12
Ankel WE (1933) Untersuchungen über Keimzellenbildung und Befruchtung bei Bithynia tentaculata. Z Zellforsch Mikrosk Anat 17:160–198
Ankel WE (1936) Prosobranchia. Tierwelt d. Nordund Ostsee Teil 9b:1–240
Austin CR (1968) Ultrastructure of Fertilization. Holt, Rhinehart and Winston, New York
Brock J (1887) Über die doppelten Spermatozoen einiger exotischer Prosobranchier. Zool Jahrb 25:615–624
Brunn M von (1884) Untersuchungen über die doppelte Form der Samenkörper von Paludina vivipara. Arch Mikr Anat 23:113–137
Buckland-Nicks JA (1973) The fine structure of the spermatozoan of Littorina (Gastropoda, Prosobranchia), with special reference to sperm motility. Z Zellforsch Mikrosk Anat 144:111–129
Buckland-Nicks JA, Chia F-S (1976) Spermatogenesis of a marine snail Littorina sitkana. Cell Tissue Res 170:455–475
Buckland-Nicks JA, Chia F-S (1981) Locomotion of the filiform spermatozoan of Littorina (Gastropoda, Prosobranchia). Cell Tissue Res 219:27–39
Buckland-Nicks JA, Williams D, Chia F-S, Fontaine A (1982a) Studies on the polymorphic spermatozoa of a marine snail. 1. Genesis of the apyrene sperm. Biol Cell (In press)
Buckland-Nicks, Williams D, Chia F-S, Fontaine A (1982b) Studies on the polymorphic spermatozoa of a marine snail. 2. Genesis of the eupyrene sperm. (In preparation)
Bulnheim HP (1962) Elektronenmikroskopische Untersuchungen zur Feinstruktur der atypischen und typischen Spermatozoen von Opalia crenimarginata (Gastropoda, Prosobranchia). Z Zellforsch Mikrosk Anat 56:300–343
Bulnheim HP (1968) Atypische Spermatozoenbildung bei Epitonium tinctum. Ein Beitrag zum Problem des Spermatozoendimorphismus der Prosobranchia. Helgol Wiss Meersunters 18:232–253
Chayen J, Bitensky L, Butcher R, Poulter L (1969) A guide to practical histochemistry. Oliver and Boyd, Edinburgh
Cloney RA, Florey E (1968) Ultrastructure of cephalopod chromatophore organs. Z Zellforsch Mikrosk Anat 89:250–280
Duval M (1879) Étude sur la spermatogenèse étudiée chez la Paludine vivipare. Rev Sci Nat S2, 1:211–231
Fain-Maurel MA (1966) Acquisitions réçentes sur les spermatogenèses atypiques. Ann Biol 5:513–564
Favard P, André J (1970) Mitochondria of spermatozoa. In: Baccetti B (ed) Comparative spermatology. Academic Press, New York, p 415
Fawcett DW (1970) A comparative view of sperm ultrastructure. Biol Rep Suppl 2:90–127
Franzen A (1955) Comparative morphological investigations into the spermiogenesis among Mollusca. Zool Bidrag Uppsala 30:399–455
Fretter V (1953) The transference of sperm from male to female prosobranch, with reference, also, to the pyramidellids. Proc Linn Soc Lond Sess 164:217–224
Furrow CL (1935) Development of the hermaphrodite genital organs of Valvata tricarinata. Z Zellforsch Mikrosk Anat 22:282–304
Gall JB (1961) Centriole replication. A study of spermatogenesis in the snail Viviparus. J Biophys Biochem Cytol 10:163–193
Garreau de Loubresse N (1971) Spermiogenesis of a prosobranch gastropod, Nerita senegalensis. Development of the intranuclear canal. J Microsc 2:425–440
Giusti F, Mazzini M (1973) The spermatozoon of Truncatella subcylindrica L. (Gastropoda, Prosobranchia). Monit Zool Ital 7:181–201
Goldschmidt R (1916) The function of the apyrene spermatozoa. Science 44:544–546
Hadfield MG (1969) Nurse eggs and giant sperm in the Vermetidae (Gastropoda). Am Zool 9:520
Healy JM, Jamieson BGM (1981) An ultrastructural examination of developing and mature paraspermatozoa in Pyrazus ebeninus (Mollusca, Gastropoda, Potamididae). Zoomorphol 98:101–119
Humason GL (1972) Animal tissue techniques, 3rd edition. WH Freeman and Co, San Francisco
Kampschmidt RF, Mayer DT, Herman HA (1953) Lipid and lipoprotein constituents of egg yolk in the resistance and storage of bull spermatozoa. J Dairy Sci 36:733–742
Kohnert R (1980) Zum Spermiendimorphismus der Prosobranchier: Spermiogenese und ultrastruktureller Aufbau der Spermien von Bithynia tentaculata (L). Zool Anz 205:145–161
Melone G, Lora Lamia Donin C, Cotelli F (1980) The paraspermatic cell (atypical spermatozoon) of Prosobranchia: A comparative ultrastructural study. Acta Zool 61:191–201
Meves F (1903) Über oligopyrene und apyrene Spermien und über ihre Entstehung nach Beobachtungen an Paludina und Pygaera. Arch Mikroskop Anat 61:1–84
Munn EA, Barnes H (1970) The fine structure of the spermatozoon of some cirripedes. J Exp Mar Biol Ecol 4:261–286
Neuhaus W (1959) Die Resorption eu-und dyspyrener Spermien bei Bithynia tentaculata. Zool Anz 163:160–168
Ohsako N (1971) Electron microscopic studies on the sperm of the freshwater snail, Radix japonica (Jay). 1. Structural characterization. Rep Fac Sci Kagoshima Univ (Earth Sci Biol) 4:63–70
Pearse AGE (1968) Histochemistry, Theoretical and Applied, vol 1. JA Churchill Ltd, London
Phillips DM (1972) Comparative analysis of mammalian sperm motility. J Cell Biol 53:561–573
Reinke EE (1912) A preliminary account of the development of apyrene sperm in Strombus and of the nurse cells in Littorina. Biol Bull 22:319–327
Reinke EE (1914) The development of the apyrene spermatozoa of Strombus bituberculatus. Carnegie Inst Wash, Pap Tortugas Lab 6:195–239
Richardson KC, Jarett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Tech 35:313
Roosen-Runge EC (1973) Germinal-cell loss in normal metazoan spermatogenesis. J Reprod Fertil 85:339–343
Roosen-Runge EC (1977) The process of spermatogenesis in animals. Camb Univ Press, London, p 145
Rothschild L, Cleland KW (1952) The physiology of sea urchin spermatozoa. The nature and localization of the endogenous substrate. J Exp Biol 29:66–71
Rousset-Galangau V (1972) Présence de deux catégories de spermies chez Limax gagates et Agriolimax agrestis (Moll Gast Pulm Lim): Étude comparée des ultrastructures au cours de la spermatogenèse. Ann Sci Nat Zool Biol Anim S12 14:319–331
Siebold CT von (1836) Fernere Beobachtungen über die Spermatozoen der wirbellosen Tiere. 2. Die Spermatozoen der Paludina vivipara. Müller's Arch Anat, Physiol Wiss Med 1:232–256
Thiriot-Quievreux C, Martoja M (1979) A propos de la notion de spermatogenèse atypique chez Carinaria lamarcki (Mollusca, Heteropoda). Neth J Zool 39:137–141
Tochimoto T (1967) Comparative histochemical study on dimorphic spermatozoa of the Prosobranchia with special reference to polysaccharides. Sci Rep Tokyo Kyoiku Daigu Ser B 13:75–109
Tuzet O (1930) Recherches sur la spermatogenèse des Prosebranches. Arch Zool Exp Gen 70:95–229
Walker M, MacGregor HC (1968) Spermatogenesis and the structure of the mature sperm of Nucella lapillus (L). J Cell Sci 3:95–104
Watson PF (1981) The roles of lipid and protein in the protection of ram spermatozoa at 5° C by egg-yolk lipoprotein. J Reprod Fertil 63:483–492
West DL (1978) Reproductive biology of Colus stimsoni II. Spermiogenesis. Veliger 21:1–9
Woodard TM (1935) Spermic dimorphism in Goniobasis laqueata. J Morphol 57:1–29
Woodard TM (1940) The function of the apyrene spermatozoa of Goniobasis laqueata Say. J Exp Zool 85:103–123
Yasuzumi G (1974) Electron microscope studies on spermiogenesis in various animal species. Int Rev Cytol 37:53–119
Yasuzumi G, Lee KJ, Fukui H, Yoshida M (1967) Spermatogenesis in animals as revealed by electron microscopy. XVII The fine structure of atypical spermatid cytoplasm of the pond snail with particularl reference to the site of hydrolytic breakdown of nuclei acids. Z Zellforsch Mikrosk Anat 80:353–369
Yasuzumi G, Yamaguchi S, Takahashi Y, Nishimura Y, Yamagishi N, Nakai Y, Naito N, Iwashita T (1975) The structural and cytochemical bases for vertebrate and invertebrate sperm motility. In: Afzelius B (ed) The functional anatomy of the spermatozoon. Pergamon Press, Oxford, p 151
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Buckland-Nicks, J., Williams, D., Chia, FS. et al. The fine structure of the polymorphic spermatozoa of Fusitriton oregonensis (Mollusca: Gastropoda), with notes on the cytochemistry of the internal secretions. Cell Tissue Res. 227, 235–255 (1982). https://doi.org/10.1007/BF00210883
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00210883