Summary
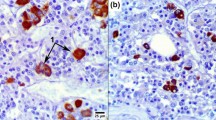
Several attempts have been made to localize steroids by means of immunocytological techniques. However, these methods were found inadequate for detecting steroids bound to their receptors. To localize endogenous testosterone (T) in its target cells at the ultrastructural level, an immunocytological technique was performed on ultrathin sections obtained by cryo-ultramicrotomy. T was detected in the pituitary glands obtained from intact male or female rats and castrated rats, but not in castrated + adrenalectomized rats. Animals were also injected either with testosterone, with other steroids (estradiol, progesterone, corticosterone) or with an androgen antagonist (cyproterone acetate). In addition, some ultrathin sections were preincubated either with phosphate buffers of various pH, corticosterone, cyproterone acetate solution, or with T solution. The content of T in the pituitary before and after fixation was measured by radioimmunoassay; it decreased after fixation. T immunoreactivity was localized in the gonadotropic cells only, both in the male and female rats. At the subcellular level, the immunoreactivity was detected in the cytoplasmic matrix and in the nucleus. Immunoreactive T disappeared 1) in rats after castration+adrenalectomy; by means of radioimmunoassay no T was measured in these pituitary glands; 2) in rats injected with 25 (μg/rat of cyproterone acetate; 3) after preincubation of pituitary sections on a drop of cyproterone acetate (1 × 10-6 M). The immunocytological reaction was not modified when the rats were injected with estradiol, progesterone or corticosterone (1 mg/rat), or after preincubation of the sections with corticosterone (1 × 10-3 M), or a buffer solution at pH 7.6. Lower or higher pH values led to a strong decrease in the immunoreactivity. After injection of T (15 μg/rat) the immunocytological reaction was more abundant in the nucleus and less in the cytoplasm. The immunoreactivity was again observed when the sections were preincubated with cyproterone acetate solution and then with T solution. These data suggest that T can be detected by means of immunocytochemistry. It is probably bound to a specific binding site.
Similar content being viewed by others
References
Castaneda E, Liao S (1975) The use of anti-steroid antibodies in the characterization of steroid receptors. J Biol Chem 250:883–888
Challis JRG, Naftolin F, Davies IJ, Ryan KJ, Lanman T (1976) Endogenous steroids in neuroendocrine tissues. In: Naftolin F, Ryan KJ, Davies J (eds) Subcellular mechanisms in reproductive neuroendocrinology. Elsevier Pub., Amsterdam, pp 247–261
Chamness GC, Mercer WD, McGuire WL (1980) Are histochemical methods for estrogen receptor valid? J Histochem Cytochem 28:792–798
Clark JH, Peck EJ, Hardin JW, Eriksson H (1978) The biology and pharmacology of estrogen receptor binding: relationship to uterine growth. In: O'Malley BW, Birnbaumer L (eds) Receptors and hormone action. Part II. Acad Press, London, pp 1–32
Coyotupa J, Parlow AF, Kovacic N (1973) Serum testosterone and dihydrotestosterone levels following orchiectomy in the adult rat. Endocrinology 92:1579–1581
Dandliker WB, Brawn RJ, Hsu ML, Brawn PN, Levin J, Meyers CY, Kolb VM (1978) Investigation of hormone-receptor interactions by means of fluorescence labelling. Cancer Res 38:4212–4217
Dubois PM, Morel G, Forest MG, Dubois MP (1978) Localization of luteinizing hormone (LH) and testosterone (T) or dihydrotestosterone (DHT) in the gonadotropic cells of anterior pituitary by using ultramicrotomy and immunocytochemistry. Horm Metab Res 10:250–252
Eriksson H, Upchurch S, Hardin JW, Peck EJ Jr, Clark JH (1978) Heterogeneity of estrogen receptors in the cytosol and nuclear fractions of the rat uterus. Biochem Biophys Res Commun 81:1–7
Fishman J, Fishman JH (1974) Competitive binding assay for estradiol receptor using immobilized antibody. J Clin Endocrinol Metab 39:603–606
Forest MG, Cathiard AM, Bertrand J (1973) Total and unbound testosterone levels in newborns and normal and hypogonadal children: use of a sensitive radioimmunoassay for testosterone. J Clin Endocrinol Metab 36:1132–1142
Forest MG, Cathiard AM, Bourgeois J, Genoud G (1974) Androgènes plasmatiques chez le nourrisson normal et prématuré. Relation avec la maturation de l'axe hypothalamo-hypophysogonadique. In: Forest MG, Bertrand J (eds) Sexual endocrinology of the perinatal period. Colloques INSERM 32:pp 315–336
Forest MG, Mappus E, Cuilleron PY (1976) O-(carboxymethyl) oxime steroidal receptors linked in the C3 position: study of the characteristics of antibodies against 17-α-hydroxyprogesterone and testosterone and of their evolution with time. Steroids, 28:815–827
Hemming FJ, Mesguich P, Morel G, Dubois PM (1983) Cryoultramicrotomy versus plastic embedding: comparative immunocytochemistry of rat anterior pituitary cells. J Microsc 131:25–34
Jensen EV, Desombre ER (1972) Mechanism of action of the female sex hormones. Ann Rev Biochem 41:203–230
Kalo J, Onouchi T (1973) 5 α-dihydrotestosterone “receptor” in the rat hypophysis. Endocrinology (Jpn) 20:641–644
King RJB, Mainwaring WIP (1974) In: King RJB, Mainwaring WIP, (eds) Steroid-cell interactions. Butterworths, London, pp 1–440
Kurzon RM, Sternberger LA (1978) Estrogen receptor immunocytochemistry. J Histochem Cytochem 26:803–808
Lieberburg I, MacLusty NJ, McEwen BS (1977) 5 α-dihydrotestosterone (DHT) receptors in rat brain and pituitary cell nuclei. Endocrinology 100:598–607
Lowry DH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–269
Mainwaring WIP (1977) Androgen receptors and biologic responses: a survey. In: O'Malley BW, Birnbaumer L (eds) Receptors and hormone action, Part II. Academic Press, NY, pp 105–120
Markaverich BM, Clark JH (1979) Two binding sites for estradiol in rat uterine nuclei: relationships to uterotropic response. Endocrinology 105:1458–1462
Morel G, Dubois PM (1982) Immunocytochemical evidence for gonadoliberin in rat anterior pituitary gland. Neuroendocrinology 34:197–206
Morel G, Forest MG, Dubois PM (1979) Spécificité tissulaire de la fixation de la testostérone: mise en évidence par immunocytochimie ultrastructurale après cryoultramicrotomie. C R Hebd Seanc Acad Sci Paris 288:1667–1670
Morel G, Forest MG, Dubois PM (1980) Etude des variations de la capture de la testostérone par les cellules gonadotropes de l'antéhypophyse du rat. C R hebd Seanc Acad Sci Paris 290:1579–1582
Morel G, Dubois PM, Benassayag C, Nunez E, Radanyi C, Redeuilh C, Richard-Foy H, Baulieu E-E (1981) Ultrastructural evidence of oestradiol receptor by immunochemistry. Exp Cell Res 132:249–258
Morel G, Besson J, Rosselin G, Dubois PM (1982) Ultrastructural evidence for endogenous vasoactive intestinal peptide-like immunoreactivity in the pituitary gland. Neuroendocrinology 34:85–89
Morel G, Hemming F, Tonon M-C, Vaudry H, Dubois MP, Coy D, Dubois PM (1983) Ultrastructural evidence for corticotropin-releasing factor (CRF)-like immunoreactivity in the rat pituitary gland. Biol Cell 44:89–92
Morel G, Mesguich P, Dubois MP, Dubois PM (1983) Ultrastructural evidence for endogenous somatostatin-like immunoreactivity in the pituitary gland. Neuroendocrinology 36:291–299
Naess O, Hansson V, Djeseland O, Attramadal A (1975) Characterization of the androgen receptor in the anterior pituitary of the rat. Endocrinology 97:1355–1363
Nenci I, Beccati MD, Piffanelli A, Lanza G (1975) Detection and dynamic localization of estradiol-receptor complexes in intact target cells by immunofluorescence technique. J Steroid Biochem 7:505–510
Nenci I, Dandliker WB, Meyers CY, Marchetti E, Marzola A, Fabris G (1980) Estrogen receptor cytochemistry by fluorescent estrogen. J Histochem Cytochem 28:1081–1088
O'Malley BW, Toft DO, Sherman MR (1971) Progesterone-binding components of chick oviduct. II Nuclear components. J Biol Chem 246:1117–1122
Pertschuk LP (1976) Detection of estrogen binding in human mammary carcinoma by immunofluorescence: a new technique utilizing the binding hormone in a polymerized state. Res Commun Chem Pathol Pharmacol 14:771–779
Robel P, Corpechot C, Baulieu E-E (1973) Testosterone and androstanolone in rat plasma and tissues. FEBS Lett 33:218–220
Sherman MR, Corvol PL, O'Malley B (1969) Progesterone-binding components of chick oviduct. I Preliminary characterization of cytoplasmic components. J Biol Chem 245:6085–6096
Stern JM, Eisenfeld AJ (1971) Distribution and metabolism of 3H-testosterone in castrated male rats; effects of cyproterone, progesterone and unlabeled testosterone. Endocrinology 88:1117–1125
Sternberger LA, Petrali JP, Joseph SA, Meyer HG, Mills KR (1978) Specificity of the immunocytochemical luteinizing hormone-releasing hormone receptor reaction. Endocrinology 102:63–73
Stumpf WE (1968) Cellular and subcellular 3H-estradiol localization in the pituitary by autoradiography. Z Zellforsch 92:23–33
Thieulant ML, Mercier L, Samperez S, Jouan P (1975) Dihydrotestosterone protein binding in the cytosol of rat hypophysis in vitro, evidence for a specific receptor. J Steroid Biochem 6:1257–1260
Tokuyasu KT (1973) A technique for ultramicrotomy of cell suspensions and tissues. J Cell Biol 57:551–565
Tymoczko JL, Liang T, Liao S (1977) Androgen receptor interactions in target cells: biochemical evaluation. In: O'Malley BW, Birnbaumer L (eds) Receptors and hormone action, Part II. Acad Press, N Y, pp 121–156
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morel, G., Forest, M.G. & Dubois, P.M. Ultrastructural evidence for endogenous testosterone immunoreactivity in the pituitary gland of the rat. Cell Tissue Res. 235, 159–169 (1984). https://doi.org/10.1007/BF00213736
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213736