Summary
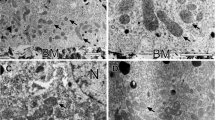
Myeloid bodies (MBs) occur in the newt (Notophthalmus viridescens) retinal pigment epithelium (RPE) and are similar to areas of specialized endoplasmic reticulum found in a variety of other cell types. The function of these structures is unknown, although a role in lipid metabolism has been strongly suggested. Random samples from conventionally-fixed and sectioned newt RPE, obtained over a 24-hr cycle (LD 12∶12), were examined by electron microscopy. Myeloid bodies appear as stacks of flattened endoplasmic reticulum-associated saccules which increase in length and number as the RPE accumulates shed outer segment material, prior to increase in the amount of stored lipid. Associations of MBs with the nuclear envelope can be related to this increased length. Myeloid bodies decrease numerically in the cell as phagosomes are removed from the cytoplasm, but a decrease in mean sectional MB area, seen in the light phase, is counteracted in darkness where individual MBs are larger than those found in the light. The total sectional area of MBs within a cell and their mean length varied depending on the lighting condition; differences were also found between eyes after extended periods of continuous light and dark. Ribosomes were found in association with the surfaces of both flattened and circular MBs, but they were consistently more densely associated with the shorter concave surfaces of curved regions. A new hypothesis for MB function is presented, which is concerned with their role in isolating toxic lipids such as retinoids, which are accumulated during phagocytosis of shed outer segment tips, and which are capable of disrupting membrane-bound systems necessary for their eventual metabolism and safe storage.
Similar content being viewed by others
References
Akahoshi T, Saito T, Yamazaki Y (1979) Ultracytochemical observation of rod outer segment phagocytosis by the retinal pigment epithelium of rat. Acta Histochem Cytochem 12:626
Amar-Costesec A, Beaufay H (1981) A structural basis of enzyme heterogeneity within liver endoplasmic retciulum. J Theor Biol 89:217–230
Arnaud J, Nobili O, Boyer J (1979) Differential properties of lipases active as membrane-bound enzymes in isolated fat cells. Biochim Biophys Acta 572:193–200
Basinger SF, Matthes MT (1980) The effect of long-term constant light on the frog pigment epithelium. Vis Res 20:1143–1149
Basinger S, Hoffman R, Matthes M (1976) Photoreceptor shedding is initiated by light in the frog retina. Science 194:1074–1076
Berman ER, Segal N, Feeney L (1979) Subcellular distribution of free and esterfied forms of Vitamin A in the pigment epithelium of the retina and the liver. Biochim Biophys Acta 572:167–177
Berman ER, Horowitz J, Segal N, Fisher S, Feeney-Burns L (1980) Enzymatic esterification of Vitamin A in the pigment epithelium of the bovine retina. Biochim Biophys Acta 630:36–46
Bibb C, Young RW (1974) Renewal of glycerol in the visual cells and pigment epithelium of the frog retina. J Cell Biol 62:378–389
Blanchette-Mackie EJ, Scow RO (1981a) Membrane continuities within cells and intercellular contacts in white adipose tissue of young rats. J Ultrastruc Res 77:277–294
Blanchette-Mackie EJ, Scow RO (1981b) Lipolysis and lamellar structures in white adipose tissue of young rats: lipid movement in membranes. J Ultrastruc Res 77:295–318
Braekevelt CR (1980) Fine structure of the retinal pigment epithelium in the mud minnow (Umbra limi). Can J Zool 58:258–276
Bradbury S, Meek GS (1958) The fine structure of the adipose cell of the leech, Glossiphonia complota. J Biophys Biochem Cytol 4:603–608
Burger PC, Herdson PB (1966) Phenobarbital-induced fine structural changes in rat liver. Am J Pathol 48:793–809
Carr I, Carr J (1962) Membranous whorls in the testicular interstitial cell. Anat Rec 144:143–147
Christensen AK, Fawcett DW (1966) The fine structure of testicular interstitial cells in mice. Am J Anat 118:551–572
Cruz-Orive LM, Weibel ER (1981) Sampling designs for stereology. J Microsc 122:235–258
Currie JR, Hollyfield JG, Rayborn ME (1978) Rod outer segments elongate in constant light. Darkness is required for normal shedding. Vis Res 18:995–1003
De Laat SW, van der Saag PT, Elson EL, Schlessinger J (1980) Lateral diffusion of membrane lipids and proteins during the cell cycle of neuroblastoma cells. Proc Natl Acad Sci 77:1526–1528
Delmelle M (1978) Retinal sensitized photodynamic damage to liposomes. Photochem Photobiol 28:357–360
Dickson DH, Hollenberg MJ (1971) The fine structure of the pigment epithelium and photoreceptor cells of the newt, Triturus viridescens dorsalis (Rafinesque). J Morphol 135:389–432
Dingle JT, Lucy JA (1962) Studies on the mode of action of excess vitamin A. 5. The effect of vitamin A on the stability of the erythrocyte membrane. Biochem J 84:611–621
Dowling JE (1960) Chemistry of visual adaptation in the rat. Nature 188:114–118
Fell HB, Dingle JT, Webb M (1962) Studies on the mode of action of vitamin A. 4. The specificity of the effect on embryonic chick-limb cartilage in culture and on isolated rat-liver lysosomes. Biochem J 83:63–69
Flight WFG, van Donselaar E (1975a) Ultrastructural aspects of the incorporation of 3H-vitamin A in the pineal organ of the urodele, Diemictylus viridescens viridescens. Proc Koninkl Neder Acad Weten 78:130–142
Flight WFG, van Donselaar E (1975b) On the effects of a prolonged osmium treatment on the ultrastructure of some cells of the pineal organ and the retina in the urodele, Diemictylus viridescens viridescens. Proc Koninkl Neder Acad Weten 78:310–324
Hendrickson AE, Kelly DE (1971) Development of the amphibian pineal organ; fine structure during maturation. Anat Rec 170:129–142
Herwig HJ (1980) Comparative ultrastructural observations on the pineal organ of the pipefish, Syngnatus acus, and the seahorse, Hippocampus hudsonius. Cell Tissue Res 209:187–200
Hollyfield JG, Basinger SF (1978) Cyclic metabolism of photoreceptor cells. Invest Ophthalmol Vis Sci 17:87–89
Jacobson K, Hou Y, Derzko Z, Wojcieszyn J, Organisciak D (1982) A comparison of lipid lateral diffusion in the cellular plasma membrane and in multilayers composed of plasma membrane lipids. Biophys J 37:8–9
Karnovsky MJ, Kleinfeld AM, Hoover RL, Klausner RD (1982) The concept of lipid domains in membranes. J Cell Biol 94:1–6
Kessel RG (1982) Differentiation of Acmaea digitalis oocytes with special reference to lipid-endoplasmic reticulum-annulate lamellae-polyribosome relationships. J Morphol 171:225–243
Kirschner DA, Hollingshead CJ (1980) Processing for electron microscopy alters membrane structure and packing in myelin. J Ultrastruc Res 73:211–232
Krinsky NI (1958) The enzymatic esterification of vitamin A. J Biol Chem 232:881–894
Kühn H (1980) Light- and GTP-regulated interaction of GTP-ase and other proteins with bovine photoreceptor membranes. Nature 283:587–589
LaVail MM (1976) Rod outer segment disc shedding in rat retina: relationship to cyclic lighting. Science 194:1071–1073
Lion F, Rotmans JP, Daemen FJM, Bonting SL (1975) Biochemical aspects of the visual process. XXVII. Stereospecficity of ocular retinol dehydrogenases and the visual cycle. Biochim Biophys Acta 384:283–292
Lo W, Bernstein MH (1981) Daily patterns of the retinal pigment epithelium. Microperoxisomes and phagosomes. Exp Eye Res 32:1–10
Marshall J, Ansell PL (1971) Membranous inclusions in the retinal pigment epithelium: phagosomes and myeloid bodies. J Anat 110:91–104
Matthes MT, Basinger SF (1980) Myeloid body associations in the frog pigment epithelium. Invest Ophthalmol Vis Sci 19:298–302
McCown JT, Evans E, Diehl S, Wiles HC (1981) Degree of hydration and lateral diffusion in phospholipid multibilayers. Biochemistry 20:3134–3138
Medline A, Bain E, Menon AI, Haberman HF (1973) Hexachlorobenzene in rat liver. Arch Pathol 96:61–65
Meeks RG, Zaharevitz D, Chen RF (1981) Membrane effects of retinoids: possible correlation with toxicity. Arch Biochem Biophys 207:141–147
Müller AE, Cruz-Orive LM, Gehr P, Weibel ER (1981) Comparison of two subsampling methods for electron microscopic morphometry. J Microsc 123:35–50
Muraoka Y, Yahara I, Nara H, Watanabe H (1981) Steroid-induced concentric membrane whorls in the dog liver. Experientia 37:389–390
Nguyen-H-Anh J (1972) Les corps myeloïdes de l'épithelium pigmentaire retinien. II. Origine et cytochimie ultrastructurale. Z Zellf 131:187–198
Nguyen-Legros J (1975) A propose des corps myeloïdes de l'épithelium pigmentaire de la retina des vértebrés. J Ultrastruc Res 53:152–163
Nguyen-Legros J (1978) Fine structure of the pigment epithelium in the vertebrate retina. Inter Rev Cytol, Suppl 7:287–328
Nistal M, Paniaqua R, Esponda P (1980) Development of the endoplasmic reticulum during human spermatogenesis. Acta Anat 108:238–249
Novikoff AB (1976) The endoplasmic reticulum: a cytochemists view (a review). Proc Natl Acad Sci 73:2781–2787
O'Day WT, Young RW (1978) Rhythmic daily shedding of outer segment membranes by visual cells in the goldfish. J Cell Biol 76:593–604
Paiement J, Godelaine D, Beaufay H (1978) Morphological changes occurring in rough microsomes from rat liver during stimulated sugar incorporation. J Cell Biol 79:221a
Porter KR (1957) The submicroscopic morphology of protoplasm. Harvey Lectures 51:175
Porter KR, Yamada E (1960) Studies on endoplasmic reticulum (ER). V. Its form and differentiation in pigment epithelial cells of frog retina. J Biophys Biochem Cytol 8:181–205
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Robinson WE, Hagins WA (1979) A light-activated GTP-ase in retinal rod outer segments. Photochem Photobiol 29:693
Rousseau A, Gatt S (1979) Interaction of membranous enzymes with membranous lipid substrates. Hydrolysis of diacylglycerol by lipase in rat liver microsomes. J Biol Chem 254:7741–7745
Ryan TA, Joiner BL, Ryan BF (1976) Minitab student handbook. Duxbury Press, North Scituate, Mass
Saari JC, Bredberg L (1982) Enzymatic reduction of 11-cis-retinal bound to cellular retinal-binding protein. Biochim Biophys Acta 716:266–272
Saari JC, Bredberg L, Garwin GG (1982) Identification of the endogenous retinoids associated with three cellular retinoid-binding proteins from bovine retina and retinal pigment epithelium. J Biol Chem 257:13329–13367
Samarasinghe DD, Petterborg LJ, Zeagler JW, Tiang KM, Reiter RJ (1983) On the occurrence of a myeloid body in pinealocytes of the white-footed mouse, Peromyscus leucopus. An electronmicroscopic study. Cell Tissue Res 228:649–660
Scow RO, Blanchette-Mackie EJ, Smith LC (1980) Transport of lipid across capillary endothelium. Fed Proc 39:2610–2617
Setoguti T, Satou Y, Goto Y (1979) Specific lamellar structures of agranular endoplasmic reticulum in the senile mouse adrenal cortex. Arch Histol Jpn 42:95–102
Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Steiner JW, Miyai K, Phillips MJ (1964) Electron microscopy of membrane-particle arrays in liver cells of ethionine-intoxicated rats. Am J Pathol 44:169–213
Stenger RJ (1966) Concentric lamellar formations in hepatic parenchymal cells of carbon tetrachloride-treated rats. J Ultrastruc Res 14:240–253
Tabor GA, Fisher SK (1983) Myeloid bodies in the mammalian retinal pigment epithelium. Invest Ophthalmol Vis Sci 24:388–391
Taira K (1981) Ultrastructural study on the whorls of rough endoplasmic reticulum in the pancreatic exocrine cells of the starved and re-fed newt. Biomed Res 2:194–201
Taira K, Mutoh H, Shibasaki S (1981) Freeze-fracture study on the whorls of rough endoplasmic reticulum in the exocrine pancreatic cells of the Japanese newt and African clawed toad. Cell Tissue Res 220:669–672
Thys O, Hildebrand J, Gerin Y, Jacques PJ (1973) Alterations of rat liver lysosomes and smooth endoplasmic reticulum induced by the diazofluoranthen derivative AC-3579. I. Morphologic and biochemical lesions. Lab Invest 28:70–82
Venkatesan S, Mitropoulos KA, Balasubramaniam S, Peters TJ (1980) Biochemical evidence for the heterogeneity of membranes from rat liver endoplasmic reticulum. Studies on the localization of acyl-CoA: cholesterol acyltransferase. Eur J Cell Biol 21:167–174
Weibel ER, Paumgartner D (1978) Integrated stereological and biochemical studies on hepatocyte membranes. II. Correction of section thickness effect on volume and surface density estimates. J Cell Biol 77:584–597
Wiggert B, Derr JE, Fitzpatrick M, Chader GJ (1979) Vitamin A receptors of the retina. Differential binding in light and dark. Biochim Biophys Acta 582:115–121
Wischnitzer S (1970) The annulate lamellae. Int Rev Cytol 27:65–100
Wu W, Huang C (1981) Effect of water mobility on lateral diffusion of phospholipids in liposomes. Lipids 16:820–822
Yamada E (1960) The fine structure of the pigment epithelium in the turtle eye. In: Smelser GK (ed) The structure of the eye. Academic Press, N.Y. pp 73–84
Young RW (1977) The daily rhythm of shedding and degradation of cone outer segment membranes in the lizard retina. J Ultrastruc Res 61:172–185
Young RW (1978) Visual cells, daily rhythms, and vision research. Vis Res 18:573–578
Zimmerman WF (1974) The distribution and proportions of vitamin A compounds during the visual cycle in the rat. Vis Res 14:795–802
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yorke, M.A., Dickson, D.H. Diurnal variations in myeloid bodies of the newt retinal pigment epithelium. Cell Tissue Res. 235, 177–186 (1984). https://doi.org/10.1007/BF00213738
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213738