Summary
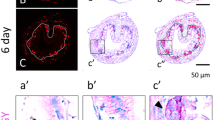
The processes of proliferation, cell division and differentiation of intestinal epithelial cells have been studied during development of the fish, Barbus conchonius. On the 3rd day, nearly all cells of the presumptive gut proliferate. Once the intestinal epithelium begins to differentiate, a decreasing percentage of proliferative cells can be found. On the 7th day, when intestinal folds start to develop, the proliferative cells become restricted to the future basal parts of the folds.
Ultrastructural examination of 3H-thymidine-labeled cells and mitotic cells of 6-day-old larvae shows that functional enterocytes are proliferative. The same feature is suggested for older fish. Proliferating undifferentiated “dark” cells, characterized by many free ribosomes and a few organelles, are also present in the intestinal epithelium of larval fish; they are considered to be stem cells, mainly for goblet cells. Proliferating goblet cells and enteroendocrine cells were not observed. The latter cell type is scarce and has a long turnover time.
A common feature of all these dividing cells is the presence of isolated spherical to cylindrical lamellar structures which may have lost contact with the cell membrane during prophase; they probably regain this contact by fusion with the cell membrane at the end of mitosis.
Similar content being viewed by others
References
Andrew A (1974) Further evidence that enterochromaffin cells are not derived from the neural crest. J Embryol Exp Morphol 31:589–598
Bjerknes M, Cheng H (1981) The stem-cell zone of the small intestinal epithelium. III: Evidence from columnar, enteroendocrine and mucous cells in the adult mouse. Am J Anat 160:77–91
Cheng H, Leblond CP (1974a) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I: Columnar cell. Am J Anat 141:461–480
Cheng H, Leblond CP (1974b) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141:537–562
Dongen JM van, Visser WJ, Daems WTh, Galjaard H (1976) The relation between cell proliferation and ultrastructural development in rat intestinal epithelium. Cell Tissue Res 174:183–200
Doyle DG (1980) The origin of nuclear bodies: a study of the undifferentiated epithelial cells of the equine small intestine. Am J Anat 157:61–70
Fontaine J, Le Douarin NM (1977) Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. The problem of the neurectodermal origin of the cells of the APUD series. J Embryol Exp Morphol 41:209–222
Fujimoto S, Hattori T, Kimoto K, Yamashita S, Fujita S, Kawai K (1980) Tritiated thymidine autoradiographic study on origin and renewal of gastrin cells in antral area of hamsters. Gastroenterology 79:785–791
Fujita T, Kobayashi S (1977) Structure and function of gut endocrine cells. In: Bourne GH, Danielli JF (eds) International review of cytology. Supplement 6, Academic Press New York pp 187–233
Gas N, Noaillac-Depeyre J (1974) Renouvellement de l'épithélium intestinal de la carpe (Cyprinus carpio L.) influence de la saison. CR Acad Sc (Paris) Sér D 279:1085–1088
Gauthier GF, Landis SC (1972) The relationship of ultrastructural and cytochemical features to absorptive activity in the goldfish intestine. Anat Rec 172:675–702
Hermos JA, Mathan M, Trier JS (1971) DNA-synthesis and proliferation by villous epithelial cells in fetal rats. J Cell Biol 50:266–258
Hyodo-Taguchi Y (1970) Effect of X-irradiation on DNA-synthesis and cell proliferation in the intestinal epithelial cells of goldfish at different temperatures with special reference to recovery process. Radiat Res 41:568–578
Iwai T (1969) Fine structure of gut epithelial cells of larval and juvenile carp during absorption of fat and protein. Arch Histol Jpn 30:183–189
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137a-138a
Lamers CHJ, Rombout JHWM, Timmermans LPM (1981) An experimental study on neural crest migration in Barbus conchonius (Cyprinidae, Teleostei) with special reference to the origin of the enteroendocrine cells. J Embryol Exp Morphol 62:309–323
Lehy TCR, Willems GMD (1976) Population kinetics of antral gastrin cells in the mouse. Gastroenterology 71:614–619
Marshall JA, Dixon KE (1978) Cell proliferation in the intestinal epithelium of Xenopus laevis tadpoles. J Exp Zool 203:31–40
McAvoy JW, Dixon KE (1977) Cell proliferation and renewal in the small intestinal epithelium in metamorphosing and adult Xenopus laevis. J Exp Zool 202:129–138
Noaillac-Depeyre J, Gas N (1973) Mise en évidence d'une zone adaptée au transport des ions dans l'intestine de carp commune (Cyprinus carpio L). CR Hebd Seance Acad Sci (Paris) 276:773–776
Noaillac-Depeyre J, Gas N (1976) Electron microscopic study on gut epithelium of the tench (Tinca tinca L.) with respect to its absorptive functions. Tissue Cell 8:511–530
Noaillac-Depeyre J, Gas N (1984) Etude cytophysiologique de l'épithélium intestinal du poisson-chat (Ameiurus nebulosus L.). Can J Zool (in press)
Pearse AGE (1973) Cell migration and the alimentary system: Endocrine contributions of the neural crest to the gut and its derivatives. General review. Digestion 8:372–385
Pearse AGE (1979) The endocrine division of the nervous system: a concept and its verification. In: Szelke McIntyre (eds) Molecular endocrinology. Elsevier, Amsterdam, pp 3–18
Rombout JHWM (1977) Enteroendocrine cells in the digestive tract of Barbus conchonius (Teleostei, Cyprinidae). Cell Tissue Res 185:435–450
Rombout JHWM, Lamers CHJ, Hanstede JG (1978) Enteroendocrine APUD cells in the digestive tract of larval Barbus conchonius (Teleostei, Cyprinidae). J Embryol Exp Morphol 47:121–135
Shcherbina MA, Trofimova LN, Kazlauskene OP (1976) The activity of protease and the resorption intensity of protein with the introduction of different quantities of fat into the food of the carp, Cyprinus carpio. J Ichthyol 16:632–636
Stroband HWJ, Dabrowski KR (1979) Morphological and physiological aspects of the digestive system and feeding in fresh-water fish larvae. In: Fontaine M. (ed) Nutrition des Poissons CNRS, pp 355–376
Stroband HWJ, Debets FMH (1978) The ultrastructure and renewal of the intestinal epithelium of the juvenile grasscarp, Ctenopharyngodon idella (Val). Cell Tissue Res 187:181–200
Stroband HWJ, Kroon AG (1981) The development of the stomach in Clarias lazera and the intestinal absorption of protein macromolecules. Cell Tissue Res 215:397–415
Stroband HWJ, Veen FH van der (1981) Localization of protein absorption during transport of food in the intestine of the grass carp, Ctenopharyngodon idella (Val). J Exp Zool 218:149–156
Stroband HWJ, Meer H vd, Timmermans LPM (1979) Regional function differentiation in the gut of the grasscarp, Ctenopharyngodon idella (Val), as determined by the alkaline phosphatase activity and the absorption of peroxidase in the intestinal epithelium. Histochemistry 64:235–249
Trier JS, Moxey PC (1980) Epithelial cell proliferation in the intestine of the winter flounder, Pseudopleuronectes americanus. Cell Tissue Res 206:379–385
Tsubouchi S (1981) Kinetic analysis of epithelial cell migration in the mouse descending colon. Am J Anat 161:239–246
Tsubouchi S, Leblond CP (1979) Migration and turnover of enteroendocrine and caveolated cells in the epithelium of the descending colon as shown by radio-autography after continuous infusion of 3H-thymidine into mice. Am J Anat 156:431–452
Vries IG de, Bosman FT (1982) Simultaneous detection of DNA-replication and hormone production using a combination of autoradiography and immunocytochemistry. J Histochem Cytochem 30:584
Yamamoto T (1966) An electron microscope study of the columnar epithelial cell in the intestine of fresh water teleosts: goldfish (Carassius auratus) and rainbow trout (Salmo irideus) Z Zellforsch 72:66–87
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rombout, J.H.W.M., Stroband, H.W.J. & Taverne-Thiele, J.J. Proliferation and differentiation of intestinal epithelial cells during development of Barbus conchonius (Teleostei, Cyprinidae). Cell Tissue Res. 236, 207–216 (1984). https://doi.org/10.1007/BF00216533
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216533