Summary
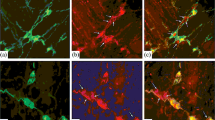
The peptides cholecystokinin (CCK), neuropeptide Y (NPY), somatostatin (SOM), substance P (SP) and vasoactive intestinal peptide (VIP), and the synthesizing enzyme for acetylcholine, choline acetyltransferase (ChAT) were localized immunohistochemically in nerve cell bodies of the submucous ganglia in the small intestine of the guinea-pig. VIP-like immunoreactivity was found in 45% of submucous neurons. ChAT immunoreactivity was observed in a separate group of nerve cells, which made up 54% of the total population. There were three subsets of neurons immunoreactive for ChAT: (1) ChAT neurons that also contained immunoreactivity for each of the peptides CCK, SOM and NPY, representing 29% of all submucous neurons; (2) ChAT neurons that also contained SP-like immunoreactivity, representing 11% of all submucous neurons, and (3) ChAT cells that did not contain any detectable amount of the peptides that were localized in this study.
Similar content being viewed by others
References
Billroth T (1858) Einige Beobachtungen über das ausgedehnte Vorkommen von Nervenanastomosen im Tractus intestinalis. Arch Anat Physiol Wiss Med 2:148–158.
Cooke HJ, Shonnard K, Wood JD (1983) Effects of neuronal stimulation on mucosal transport in guinea pig ileum. Am J Physiol 245:G290-G296.
Costa M, Furness JB (1982) Neuronal peptides in the intestine. Brit Med Bull 38:247–252.
Costa M, Furness JB (1983) The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guineapig small intestine. Neuroscience 8:665–676.
Costa M, Furness JB (1984) Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience (submitted).
Costa M, Buffa R, Furness JB, Solcia EL (1980) Immunohistochemical localization of polypeptides in peripheral autonomic nerves using whole mount preparations. Histochemistry 65:157–165.
Costa M, Furness JB, McLean JR (1976) The presence of aromatic l-amino acid decarboxylase in certain intestinal nerve cells. Histochemistry 48:129–143.
Costa M, Patel Y, Furness JB, Arimura A (1977) Evidence that some intrinsic neurons of the intestine contain somatostatin. Neurosci Lett 6:215–222.
Costa M, Furness JB, Llewellyn-Smith IJ, Davies B, Oliver J (1980) An immunohistochemical study of the projections of somatostatin-containing neurons in the guinea-pig intestine. Neuroscience 5:841–852.
Costa M, Furness JB, Llewellyn-Smith IJ, Cuello A (1981) Projections of substance P neurons within the guinea-pig small intestine. Neuroscience 6:411–424.
Cuello AC, Galfre G, Milstein C (1979) Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 67:3532–3536.
Dockray GJ, Williams RG, Zhu W-Y (1981) Development of region-specific antisera for the C-terminal tetrapeptide of gastrin/cholecystokinin and their use in studies of immunoreactive forms of cholecystokinin in rat brain. Neurochem Int 3:281–288.
Eckenstein F, Barde YA, Thoenen H (1981) Production of specific antibodies to choline acetyltransferase purified from pig brain. Neuroscience 6:993–1000.
Eckenstein F, Thoenen H (1982) Production of specific antisera and monoclonal antibodies to choline acetyltransferase: characterization and use for identification of cholinergic neurons. EMBO J 1:363–368.
Eskay RL, Furness JB, Long RT (1981) Substance P activity in the bullfrog retina: localization and identification in several vertebrate species. Science 212:1049–1051.
Furness JB, Costa M (1978) Distribution of intrinsic nerve cell bodies and axons which take up aromatic amines and their precursors in the small intestine of the guinea-pig. Cell Tissue Res 188:527–543.
Furness JB, Costa M, Walsh JH (1981) Evidence for and significance of the projection of VIP neurons from the myenteric plexus to the taenia coli in the guinea-pig. Gastroenterology 80:1557–1561.
Furness JB, Costa M, Eckenstein F (1983a) Neurons localized with antibodies against choline acetyltransferase in the enteric nervous system. Neurosci Lett 40:105–109.
Furness JB, Costa M, Emson PC, Håkanson R, Moghimzadeh E, Sundler F, Taylor IL, Chance RE (1983b) Distribution, pathways and reactions to drug treatment of nerves with neuropeptide Y and pancreatic polypeptide-like immunoreactivity in the guinea-pig digestive tract. Cell Tissue Res 234:71–92.
Gaginella TS, O'Dorisio TM (1979) Vasoactive intestinal polypeptide: neuromodulator of intestinal secretion? In: Bind HJ (ed) Mechanisms of intestinal secretion. Alan R. Liss, New York, p 231–237.
Gilbert RFT, Emson PC (1983) Neuronal coexistence of peptides with other putative transmitters. In: Iversen L, Iversen S, Snyder S (eds) Handbook of psychopharmacology: Volume 16, Neuropeptides. Plenum Press, New York, London, p 519–556.
Hirst GDS, McKirdy HC (1975) Synaptic potentials recorded from neurones of the submucous-plexus of guinea-pig small intestine. J Physiol 249:369–385.
Hirst GDS, Silinsky EM (1975) Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol 251:817–832.
Hökfelt T, Lundberg J, Schultzberg M, Johansson O, Ljungdahl A, Rehfeld J (1980) Coexistence of peptides and putative transmitters in neurons. In: Costa E, Trabucchi M (eds) Neural peptides and neuronal communication: Vol 22, Advances in biomechemical psychopharmacology. Raven Press, New York, p 1–23.
Johansson O, Hökfelt T, Pernow B, Jeffcoate SL, White N, Steinbusch HWM, Verhofstad AAJ, Emson PC, Spindel E (1981) Immunohistochemical support for three putative transmitters in one neuron: coexistence of 5-hydroxytryptamine, substance P-, and TRH-like immunoreactivity in medullary neurons projecting to the spinal cord. Neuroscience 6:1857–1881.
Keast JR, Furness JB, Costa M (1984) The origins of peptide and norepinephrine nerves in the mucosa of the guinea-pig small intestine. Gastroenterology 86:637–644.
Lundberg JM, Hökfelt T, Anggard A, Kimmel J, Goldstein M, Markey K (1980) Coexistence of an avian pancreatic polypeptide (APP) immunoreactive substance and catecholamines in some peripheral and central neurons. Acta Physiol Scand 110:107–109.
Meissner G (1857) Über die Nerven der Darmwand. Z Rat Med 8:364–366.
Schofield GC (1968) Anatomy of muscular and neural tissues in the alimentary canal. Handbook of physiology: Alimentary Canal Sect 6 Vol 4:1579–1627.
Schultzberg M, Hökfelt T, Nilsson G, Terenius L, Rehfeld JF, Brown M, Elde R, Goldstein M, Said S (1980) Distribution of peptide- and catecholamine-containing neurons in the gastrointestinal tract of rat and guinea-pig: Immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine β-hydroxylase. Neuroscience 5:689–744.
Sundler F, Moghimzadeh E, Håkanson R, Ekelund M, Emson P (1983) Nerve fibres in the gut and pancreas of the rat displaying neuropeptide-Y immunoreactivity: Intrinsic and extrinsic origin. Cell Tissue Res 230:487–493.
Tapper EJ (1983) Local modulation of intestinal ion transport by enteric neurons. Am J Physiol 244:G457-G468.
Vaillant C, Taylor IL (1981) Demonstration of carboxyl-terminal PP-like peptides in endocrine cells and nerves. Peptides 2:31–35.
Wilson AJ, Furness JB, Costa M (1981a) The fine structure of the submucous plexus of the guinea-pig ileum. I. The ganglia, neurons, Schwann cells and neuropil. J Neurocytol 10:759–784.
Wilson AJ, Furness JB, Costa M (1981b) The fine structure of the submucous plexus of the guinea-pig ileum. II. Description and analysis of vesiculated nerve profiles. J Neurocytol 10:785–804.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Furness, J.B., Costa, M. & Keast, J.R. Choline acetyltransferase- and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res. 237, 329–336 (1984). https://doi.org/10.1007/BF00217152
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217152