Summary
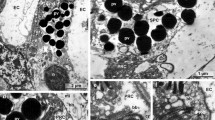
The cone cells and corneagenous cells possess extensive networks of smooth tubular endoplasmic reticulum that may be involved in optical reflectance and light-adaptational responses, respectively. The extracellular basal lamina of the basement membrane is confluent with glial cell capillary walls and may prove to be a viaduct for the transmission of hemolymph-borne substances to the retina or of retinal degradation products to the hemolymph. In addition to dense pigment granules, the distal pigment cells are shown for the first time to contain migratory reflecting platelets that are usually polymorphic in light-adapted eyes but are rectangular in dark-adapted eyes. In the latter these plates become aligned against the crystalline cones and presumably contribute to the reflection superposition optics of the grass shrimp. Dark-adapted retinular cells possess well-developed perirhabdomal cisternae, oblong or ovoid mitochondria, generally vesicular rough endoplasmic reticulum, and occasional, spherical, calcium-like intrarhabdomal inclusions. Light-adapted retinular cells possess poorly developed perirhabdomal cisternae, lamelliform rough endoplasmic reticulum, and condensed mitochondria frequently associated with lipid droplets and pigment granules. The cytoplasmic boundaries of the reflecting pigment cells expand into the extracellular spaces between individual ommatidial retinular cells during dark adaptation and recede to the interommatidial extracellular spaces during light adaptation. Cytoplasmic microfilament bundles found only at the bases of partially light-adapted rhabdomeric microvilli may be involved in microvillar shortening.
Similar content being viewed by others
References
Bell AL, Barnes SN, Anderson KL (1969) A fixation technique for electron microscopy which provides uniformly good preservation of the tissues of a variety of marine invertebrates. Biol Bull 173:393
Blest AD, DeCouet HG (1983) Actin in cellular components of the basement membrane of the compound eye of a blowfly. Cell Tissue Res 231:325–336
Blest AS, Stowe S, Price DG (1980) The sources of acid hydrolases for photoreceptor membrane degradation in a grapsid crab. Cell Tissue Res 205:229–244
Blest AD, Stowe S, Eddy W, Williams DS (1982) The local deletion of a microvillar cytoskeleton from photoreceptors of tipulid flies during membrane turnover. Proc R Soc Lond B 215:469–479
Bonneville MA, Weinstock M (1970) Brush border development in the intestinal absorptive cells of Xenopus during metamorphosis. J Cell Biol 44:151–171
Butman BT, Obika M, Tchen TT, Taylor JD (1979) Hormone induced pigment translocations in amphibian dermal iridophores. In vitro: Changes in cell shape. J Exp Zool 208:17–33
Byers HR, Porter KR (1977) Transformations in the structure of the cytoplasmic ground substance in erythrophores during pigment aggregation and dispersion. J Cell Biol 75:541–558
Debaisieux P (1944) Les yeux des Crustacés-Structure, developpement, réactions a l'éclairement. La Cellule 50:9–122
Eguchi E, Waterman TH (1967) Changes in retinal fine structure induced in the crab Libinia by light and dark adaptation. Z Zellforsch 79:209–229
Elofsson R, Hallberg E (1973) Correlation of ultrastructure and chemical composition of crustacean chromatophore pigment. J Ultrastruct Res 44:421–429
Elofsson R, Odselius R (1975) The anostracan rhabdom and the basement membrane. An ultrastructural study of the Artemia compound eye (Crustacea). Acta Zool 56:141–153
Exner S (1891) Die Physiologie der facettierten Augen von Krebsen und Insekten. Franz Deuticke, Leipzig Wien
Fincham AA (1980) Eyes and classification of malacostracan crustaceans. Nature 287:729–731
Fingerman M, Lowe ME, Sundararaj BI (1959) Dark adapting and light adapting hormones controlling the distal retinal pigment of the prawn Palaemonetes vulgaris. Biol Bull 116:30–36
Fingerman M, Nagabhushanam R, Philpott L (1962) Photomechanical responses of the proximal pigment in Palaemonetes and Orconectes. Biol Bull 123–131
Frixione E (1983) Firm structural associations between migratory pigment granules and microtubules in crayfish retinula cells. J Cell Biol 96:1258–1265
Frixione E, Aréchiga H (1979) Photomechanical migrations of pigment granules along the retinula cells of the crayfish. J Neurobiol 10:573–590
Frixione E, Aréchiga H (1981) Ionic dependence of screening pigment migrations in crayfish retinal photoreceptors. J Comp Physiol 144:35–43
Frixione E, Tsutsumi V (1982) Photomechanical responses in crustacean retinula cells: the role of microtubules. Vision Res 22:1507–1514
Hafner GS, Tokarski T, Hammond-Soltis G (1982) Development of the crayfish retina: a light and electron microscopic study. J Morphol 173:101–118
Hallberg E (1977) The fine structure of the compound eyes of mysids (Crustacea: Mysidacea). Cell Tissue Res 184:45–65
Hallberg E (1978) The compound eye of crustaceans and its screening pigment. Thesis, University of Lund
Itaya SK (1976a) Rhabdom changes in the shrimp Palaemonetes. Cell Tissue Res 166:265–273
Itaya SK (1976b) Light and dark adaptational changes in the accessory eye of the shrimp Palaemonetes. Tissue Cell 8:583–590
Kirschfeld K, Vogt K (1980) Calcium ions and pigment migration in fly photoreceptors. Naturwissenschaften 67:516
Kleinholz LH (1936) Crustacean eye-stalk hormone and retinal pigment migration. Biol Bull 70:159–184
Kleinholz LH (1959) Purines and pteridines from the reflecting pigment of the arthropod retina. Biol Bull Mar Biol Lab 116:125–135
Kleinholz LH (1961) Pigmentary effectors. In: Waterman TH (ed) The physiology of crustacea, Vol II. Academic Press, New York, pp 133–169
Kleinholz LH (1966) Hormonal regulation of retinal pigment migration in crustaceans. In: Bernhard CG (ed) The functional organization of the compound eye. Pergamon, Oxford, pp 89–101
Kuiper JW (1962) The optics of the compound eye. In: Beament JWL (ed) Biological receptor mechanisms. University Press Cambridge, Symp Soc Exp Biol 16:58–71
Land MF (1972) The physics and biology of animal reflectors. In: Butler JAV, Noble D (eds) Progress in biophysics and molecular biology, Vol 24. Pergamon, New York, pp 77–106
Land MF (1976) Superposition images are formed by reflection in the eyes of some oceanic decapod crustacea. Nature 263:764–765
Land MF (1980) Compound eyes: old and new optical mechanisms. Nature 287:681–686
Land MF (1981) Optics and vision in invertebrates. In: Autrum H (ed) Handbook of sensory physiology, Vol VII/6B. Springer, Berlin Heidelberg New York, pp 471–592
Leggett LMW, Stavenga DG (1981) Diurnal changes in angular sensitivity of crab photoreceptors. J Comp Physiol 144:99–109
Menter DG, Obika M, Tchen TT, Taylor JD (1978) Leucophores and iridophores of Fundulus heteroclitus: biophysical and ultrastructural characteristics. J Morphol 160:103–120
Nagano T (1950) Physiological studies on the pigmentary system of Crustacea IV. Studies on the diurnal rhythm of the eye pigments of the shrimps. Science Repts Tohoku Univ Fourth Series 18:286–297
Nassel DR, Waterman TH (1979) Massive diurnally modulated photoreceptor membrane turnover in crab light and dark adaptation. J Comp Physiol 131:205–216
Nilsson DE (1983) Evolutionary links between apposition and superposition optics in crustacean eyes. Nature 302:818–821
Odselius R, Elofsson R (1981) The basement membrane of the insect and crustacean compound eye: definition, fine structure, and comparative morphology. Cell Tissue Res 216:205–214
Olivo RF, Larsen ME (1978) Brief exposure to light initiates screening pigment migration in retinula cells of the crayfish Procambarus. J Comp Physiol A 125:91–96
Parker GH (1891) The compound eyes in crustaceans. Bull Mus Comp Zool 21:45–142
Parker GH (1897) Photomechanical changes in the retinal pigment cells of Palaemonetes and their relations to the central nervous system. Bull Mus Comp Zool 30:275–300
Piekos WB, Waterman TH (1983) Nocturnal rhabdom cycling and retinal hemocyte functions in crayfish (Procambarus) compound eyes I. Light microscopy. J Exp Zool 225:209–217
Rao KR (1984) Pigmentary effectors. In: Bliss DE, Mantel LH (eds) The biology of Crustacea, Vol 9. Academic Press, New York, pp 395–462
Rao KR, Doughtie DG, Robinson PA, Riehm JP (1983) Effects of synthetic Pandalus DRPH on the ommatidial pigmentary effectors in Palaemonetes pugio. Am Zool 23:951
Rodewald R, Newman SB, Karnovsky MJ (1976) Contraction of isolated brush borders from the intestinal epithelium. J Cell Biol 70:541–554
Schliwa M (1979) Stereo high voltage electron microscopy of melanophores. Matrix transformations during pigment movements and the effects of cold and colchicine. Exp Cell Res 118:323–340
Snyder AW, Horridge GA (1972) The optical function of changes in the medium surrounding the cockroach rhabdom. J Comp Physiol 81:1–8
Stowe S (1980) Rapid synthesis of photoreceptor membrane and assembly of new microvilli in a crab at dusk. Cell Tissue Res 211:419–440
Stowe S (1981) Effects of illumination changes on rhabdom synthesis in a crab. J Comp Physiol 142:19–25
Toh Y, Waterman TH (1982) Diurnal changes in compound eye fine structure in the blue crab Callinectes. J Ultrastruct Res 78:40–59
Tsutsumi V, Frixione E, Aréchiga H (1981) Transformations in the cytoplasmic structure of crayfish retinula cells during light and dark adaptation. J Comp Physiol A 145:179–189
Vogt K (1975) Zur Optik des Flußkrebsauges. Z Naturforsch 30:691
Vogt K (1977) Ray path and reflection mechanisms in crayfish eyes. Z Naturforsch 32c:466–468
Vogt K (1980) Die Spiegeloptik des Flußkrebsauges. J Comp Physiol 135:1–19
Walz B (1982) Calcium sequestering smooth endoplasmic reticulum in retinula cells of the blowfly. J Ultrastruct Res 81:240–248
Waterman TH (1961) Light sensitivity and vision. In: Waterman TH (ed) The physiology of Crustacea Vol II. Academic Press New York, pp 1–64
Waterman TH, Piekos WB (1981) Light and time correlated migration of invasive hemocytes in the crayfish compound eye. J Exp Zool 217:1–14
Waterman TH, Piekos WB (1983) Nocturnal rhabdom cycling and retinal hemocyte functions in crayfish (Procambarus) compound eyes. II. Transmission electron microscopy and acid phosphatase localization. J Exp Zool 225:219–231
Welsh JH (1930) The mechanics of migration of the distal pigment cells in the eyes of Palaemonetes. J Exp Zool 56:459–494
Welsh JH (1941) The sinus glands and 24-h cycles of retinal pigment migration in the crayfish. J Exp Zool 86:35–49
Zyznar ES, Nicol JAC (1971) Ocular reflecting pigments in some Malacostraca. J Exp Mar Biol Ecol 6:235–248
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doughtie, D.G., Rao, K.R. Ultrastructure of the eyes of the grass shrimp, Palaemonetes pugio . Cell Tissue Res. 238, 271–288 (1984). https://doi.org/10.1007/BF00217299
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217299