Summary
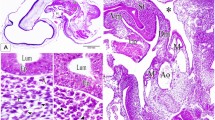
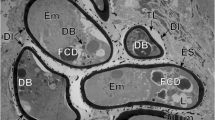
The endolymphatic sac of the tree frog and its crystals were observed by light- and electron microscopy. Scanning electron microscopy revealed that the crystals have a faceted body and two pointed ends. Light- and transmission electron microscopy revealed that the endolymphatic sac is composed of many small chambers. In their lumina, numerous “ghosts” of crystals that resulted from decalcification were observed. The ghosts were demarcated by a linear dense material or embedded in a flocculent substance. The epithelium of the endolymphatic sac is simple squamous or cuboidal and peculiar cytoplasmic granules are found in most cells. The granules are surrounded by a limiting membrane and have varying electron density. Some granules contain a core and/or tubular structures. Vacuoles containing large ghosts are also found in the epithelial cells. These ghosts were quite similar to those in the lumen and sometimes coexist with cell debris. The fine structure of the endolymphatic sac and its crystals is discussed.
Similar content being viewed by others
References
Anniko M, Ylikoski J, Wróblewski R (1984) Microprobe analysis of human otoconia. Acta Otolaryngol (Stockh) 97:283–289
Ballarino J, Howland HC (1982) Otoconial morphology of the developing chick. Anat Rec 204:83–87
Balsamo G, De Vincentiis M, Marmo F (1969a) The effect of tetracyclin on the processes of calcification of the otoliths in the developing chick embryo. J Embryol Exp Morphol 22:327–332
Balsamo G, De Vincentiis M, Marmo F, Parisi G (1969b) The effect of 4,5-dichlor-1,3-benzendisulfonamide and of 4,5-dichlor-1,3-benzendisulfonylaniline on the morphogenesis of the otoliths in the chick embryo. Experientia 25:292–293
Bélanger LF (1960) Development, structure and composition of the otolithic organs of the rat. In: Sognnaes RF (ed) Calcification in biological systems. American Association for the Advancement of Science, Washington, pp 151–162
Carlström D (1963) A crystallographic study of vertebrate otoliths. Biol Bull 125:441–463
Carlström D, Engström (1955) The ultrastructure of statoconia. Acta Otolaryngol (Stockh) 45:14–18
Carlström D, Engström, Hjorth S (1953) Electron microscopic and X-ray diffraction studies of statoconia. Laryngoscope 63:1052–1057
Ciges M, Campos A, Cañizares J, Crespo P (1985) Otoconial mineralization in post-natal development. Acta Otolaryngol (Stockh) 99:399–404
Ciges M, Campos A, Revelles F (1983) The origin of the otoconia in the rat. Acta Otolaryngol (Stockh) 95:522–527
Dempsler WT (1930) The morphology of the amphibian endolymphatic organ. J Morphol 50:71–126
De Vincentiis M, Marmo F (1968) Inhibition of the morphogenesis of the otoliths in the chick embryo in the presence of carbonic anhydrase inhibitors. Experientia 24:818–820
Dietrich HE, Fontaine AR (1975) A decalcification method for ultrastructure of echinoderm tissues. Stain Technol 50:351–354
Fermin CD, Igarashi M (1985) Development of otoconia in the embryonic chick (Gallus domesticus). Acta Anat 123:148–152
Funaoka S, Toyota S (1928) Untersuchungen über die transmikroskopische Struktur des Lebewesenkörpers. III. Über die chemische Natur und die Entstehung der Otolithen der Rana esculenta. Folia Anat Jpn 6:323–325
Hastings AB (1935) Chemical analysis of otoliths and endolymphatic sac deposits in Amblystoma tigrinum. J Comp Neurol 61:295–296
Hunter-Duvar IM (1983) An electron microscopic study of the vestibular sensory epithelium. Acta Otolaryngol (Stockh) 95:494–507
Imoto T, Rask-Andersen H, Bagger-Sjöbäck D (1983) The role of the endolymphatic sac in statoconial formation and degradation. Acta Otolaryngol (Stockh) 96:227–235
Kimura R, Lundquist P, Wersäll J (1964) Secretory epithelial linings in the ampullae of the guinea pig labyrinth. Acta Otolaryngol (Stockh) 57:517–530
Lim DJ (1973) Formation and fate of the otoconia. Scanning and transmission electron microscopy. Ann Otol Rhinol Laryngol 82:23–35
Lim DJ (1974) The statoconia of the non-mammalian species. Brain Behav Evol 10:37–51
Lundquist P-G (1965) The endolymphatic duct and sac in the guinea pig. An electron microscopic and experimental investigation. Acta Otolaryngol (Stockh) [Suppl] 201:1–108
Marmo F, Franco E, Balsamo G (1981) Scanning electron microscopic and X-ray diffraction studies of otoconia in the lizard Podarcis s. sicula. Cell Tissue Res 218:265–270
Nakahara H, Bevelander G (1979) An electron microscope study of crystal calcium carbonate formation in the mouse otolith. Anat Rec 193:233–242
Ross MD, Peacor DR (1975) The nature and crystal growth of otoconia in the rat. Ann Otol Rhinol Laryngol 84:22–36
Ross MD, Johnsson L-G, Peacor D, Allard LF (1976) Observations on normal and degenerating human otoconia. Ann Otol Rhinol Laryngol 85:310–326
Sánchez-Fernández JM, Rivera-Pomar JM (1984) A scanning electron microscopy study on human otoconia genesis. Acta Otolaryngol (Stockh) 97:479–488
Schlumberger HG, Burk DH (1953) Comparative study of the reaction to injury. II. Hypervitaminosis D in the frog with special reference to the lime sacs. AMA Arch Pathol 56:103–124
Vasquez CS (1955) Calcareous formations in the endolymphatic sac of chicken embryos. Ann Otol Rhinol Laryngol 64:1019–1024
Verhage HG, Bareither ML, Jaffe RC, Akbar M (1979) Cyclic changes in ciliation, secretion and cell height of the oviductal epithelium in women. Am J Anat 156:505–522
Vilstrup T (1951) On the formation of the otoliths. Ann Otol Rhinol Laryngol 60:974–981
Wright CG, Lee DH (1986) Pigmented epithelial cells of the membranous saccular wall of the chinchilla. Acta Otolaryngol (Stockh) 102:438–449
Yamane H, Imoto T, Nakai Y, Igarashi M, Rask-Andersen H (1984) Otoconia degradation. Acta Otolaryngol (Stockh) [Suppl] 406:263–270
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawamata, S., Takaya, K. & Yoshida, T. Light- and electron-microscopic study of the endolymphatic sac of the tree frog, Hyla arborea japonica . Cell Tissue Res. 249, 57–62 (1987). https://doi.org/10.1007/BF00215418
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00215418