Summary
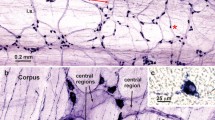
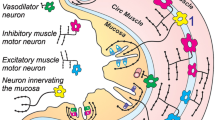
Galanin immunoreactivity was observed in nerve cell bodies and nerve fibres, but not in enteroendocrine cells, in the small intestine of the guinea-pig. Nerve terminals were found in the myenteric plexus, in the circular muscle, in submucous ganglia, around submucous arterioles, and in the mucosa. Lesion studies showed that all terminals were intrinsic to the intestine; those in myenteric ganglia arose from cell bodies in more orally placed ganglia. Myenteric nerve cells were also the source of terminals in the circular muscle. Galanin (GAL) was located in a population of submucous nerve cell bodies that also showed immunoreactivity for vasoactive intestinal peptide (VIP) and in a separate population that was immunoreactive for neuropeptide Y (NPY). Processes of the GAL/VIP neurons supplied submucous arterioles and the mucosal epithelium. Processes of GAL/NPY neurons ran to the mucosa. It is concluded that galanin immunoreactivity occurs in several functionally distinct classes of enteric neurons, amongst which are neurons controlling (i) motility, (ii) intestinal blood flow, and (iii) mucosal water and electrolyte transport.
Similar content being viewed by others
References
Bauer FE, Adrian TE, Christofides ND, Ferri G-L, Yanaihara N, Polak JM, Bloom SR (1986) Distribution and molecular heterogeneity of galanin in human, pig, guinea-pig and rat gas-trointestinal tracts. Gastroenterology 91:833–877
Bornstein JC, Costa M, Furness JB, Lees GM (1984) Electrophysiology and enkephalin immunoreactivity of identified myenteric plexus neurons of guinea-pig small intestine. J Physiol (Lond) 351:313–325
Bornstein JC, Costa M, Furness JB (1986) Synaptic input to immunohistochemically identified neurons in the submucous plexus of the guinea-pig small intestine. J Physiol (Lond) 381:465–482
Bywater RAR, Taylor GS (1986) Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. J Physiol (Lond) 374:153–164
Cassuto J, Fahrenkrug J, Jodal M, Tuttle R, Lundgren O (1981) The release of vasoactive intestinal polypeptide from the cat small intestine exposed to cholera toxin. Gut 22:958–963
Cooke HJ (1984) Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol 248:G263-G267
Costa M, Furness JB (1983) The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guineapig small intestine. Neuroscience 8:665–676
Costa M, Furness JB (1983) Immunohistochemistry on whole mount preparations. In: Cuello AC (ed) Immunohistochemistry IBRO Handbook Series, Methods in the Neuroscience. Wiley and Sons, Chichester
Costa M, Furness JB, Cuello AC (1985a) Separate populations of opioid containing neurons in the guinea-pig intestine. Neuropeptides 5:445–448
Costa M, Furness JB, Pullin CO, Bornstein J (1985b) Substance P enteric neurons mediate non-cholinergic transmission to the circular muscle of the guinea-pig intestine. Naunyn-Schmiedeberg's Arch Pharmacol 328:446–453
Costa M, Furness JB, Gibbins IL (1986a) Chemical coding of enteric neurons. In: Hökfelt T, Changeux P Progress in Brain Research. Elsevier, Amsterdam 68:217–240
Costa M, Furness JB, Humphreys CMS (1986b) Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn Schmideberg's Arch Pharmacol 332:79–88
Cuello AC, Galfre G, Milstein C (1979) Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 76:3532–3536
Dogiel AS (1899) Über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere. Arch Anat Physiol Leipzig, Anat Abt:130–158
Ekblad E, Rökaeus Å, Håkanson R, Sundler F (1985a) Galanin nerve fibres in the rat gut: distribution, origin and projections. Neuroscience 16:355–363
Ekblad E, Håkanson R, Sundler F, Wahlestedt C (1985b) Galanin: neuromodulatory and direct contractile effects on smooth muscle preparations. J Pharmacol 86:241–246
Fahrenkrug J, Haglund U, Jodal M, Lundgren O, Olbe L, Schaffanlitzky de Muckadell OB (1978) Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J Physiol (Lond) 284:291–305
Fox JET, McDonald TJ, Kostolanska F, Tatemoto K (1986) Galanin: an inhibitory neuronal peptide of the canine small intestine. Life Sci 39:103–110
Furness JB, Costa M (1978) Distribution of intrinsic nerve cell bodies and axons which take up aromatic amines and their precursors in the small intestine of the guinea-pig. Cell Tissue Res 188:527–543
Furness JB, Costa M (1979) Projections of intestinal neurons showing immunoreactivity for vasoactive intestinal polypeptide are consistent with these neurons being the enteric inhibitory neurons. Neurosci Lett 15:199–204
Furness JB, Costa M (1987) The enteric nervous system. Churchill Livingstone, Edinburgh, pp 287
Furness JB, Costa M, Emson PG, Håkanson R, Maghimzadeh E, Sundler F, Taylor IL, Chance RE (1983) Distribution, pathways and reactions to drug treatment of nerves with neuropeptide Y and pancreatic polypeptide-like immunoreactivity in the guinea pig digestive tract. Cell Tissue Res 234:71–92
Furness JB, Costa M, Keast JR (1984) Choline acetyltransferase and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res 237:328–336
Furness JB, Costa M, Gibbins IL, Llewellyn-Smith IJ, Oliver JR (1985) Neurochemically similar myenteric and submucous neurons directly traced to the mucosa of the small intestine. Cell Tissue Res 241:155–163
Furness JB, Costa M, Morris JL, Gibbins IL (1987) Novel neurotransmitters and the chemical coding of neurons. Advances Physiol Res, Plenum Press, New York, pp 143–165
Katayama Y, Lees GM, Pearson GT (1986) Electrophysiology and morphology of vasoactive-intestinal-peptide-immunoreactive neurons of the guinea pig ileum. J Physiol (Lond) 378:1–11
Keast JR (1987) Mucosal innervation and control of water and ion transport in the intestine. Reviews in Physiology, Biochemistry and Pharmacology. Springer, Berlin Heidelberg New York (in press)
Keast JR, Furness JB, Costa M (1984) The origins of peptide and norepinephrine nerves in the mucosa of the guinea-pig small intestine. Gastroenterology 86:637–644
Keast JR, Furness JB, Costa M (1985) Investigations of nerve populations influencing ion transport that can be stimulated electrically, by serotonin and by a nicotinic agonist. Naunyn Schmiedeberg's Arch Pharmacol 331:260–266
Kishimoto S, Kajiyama G, Miyoshi A (1986) Comparative study on distribution of galanin and vasoactive intestinal polypeptide (PHM) in human gut, gallbladder and pancreas. Can J Physiol Pharmacol, Gastrointestinal Hormones Supplement, p 156
Melander T, Hökfelt T, Rokaeus A, Fahrenkrug J, Tatemoto K, Mutt V (1985) Distribution of galanin-like immunoreactivity in the gastro-intestinal tract of several mammalian species. Cell Tissue Res 239:253–270
Morris JL, Gibbins IL, Furness JB, Costa M, Murphy R (1985) Co-localization of NPY, VIP and dynorphin in non-noradrenergic axons of the guinea pig uterine artery. Neurosci Lett 62:31–37
Morris JL, Gibbins IL, Murphy R (1986) Neuropeptide Y-like immunoreactivity is absent from most perivascular noradrenergic axons in a marsupial, the brush-tailed possum. Neurosci Lett 71:264–270
Rökaeus A, Brownstein MJ (1986) Construction of a porcine adrenal medullary cDNA library and nucleotide sequence analysis of two clones encoding a galanin precursor. Proc Natl Acad Sci US A 83:6287–6291
Rökaeus A, Melander T, Hökfelt T, Lundberg JM, Tatemoto K, Carlquist M, Mutt V (1984) A galanin-like peptide in the central nervous system and intestine of the rat. Neurosci Lett 47:161–166
Tatemoto K, Rökaeus A, Jornvall H, McDonald T, Mutt V (1983) Galanin — a novel biologically active peptide from porcine intestine. FEBS Lett 164:124–128
Wessendorf MW, Elde RP (1985) Characterization of an immunofluorescence technique for the demonstration of coexisting neurotransmitters within nerve fibres and terminals. J Histochem Cytochem 33:984–994
Wilson AJ, Furness JB, Costa M (1981a) The fine structure of the submucous plexus of the guinea-pig ileum I. The ganglia, neurons, Schwann cells and neuropil. J Neurocytol 10:759–784
Wilson AJ, Furness JB, Costa M (1981b) The fine structure of the submucous plexus of the guinea-pig ileum II. Description and analysis of vesiculated nerve profiles. J Neurocytol 10:785–804
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Furness, J.B., Costa, M., Rökaeus, Å. et al. Galanin-immunoreactive neurons in the guinea-pig small intestine: their projections and relationships to other enteric neurons. Cell Tissue Res. 250, 607–615 (1987). https://doi.org/10.1007/BF00218954
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00218954