Summary
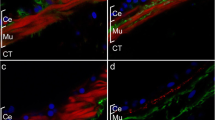
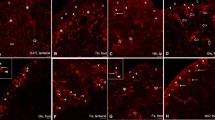
The distribution, morphology and synaptic connections of the hindgut efferent neurons in the last (sixth) abdominal ganglion of the crayfish, Orconectes limosus, have been investigated using light and electron microscopy in conjunction with retrograde cobalt/nickel and HRP labeling through the intestinal nerve. The hindgut efferent neurons occur singly and in clusters, and are unipolar. Their axonal projections are uniform and consist of a thick primary neurite with typical lateral projections and limited arborization of varicose fibers in the ganglionic neuropil. They also send lower order axon processes to the ganglionic neural sheath, where they arborize profusely, forming a network of varicose fibers. The majority of the efferent neurons project to the anterior part of the hindgut. HRP-labeled axon profiles are found in both pre- and postsynaptic position in the neuropil of the ganglion. HRP-labeled axon profiles also establish pre- and postsynaptic contacts in the intestinal nerve root. All hindgut efferent terminals contain similar synaptic vesicle populations: ovoid agranular vesicles (50–60 nm) and a few large granular vesicles (100–200 nm). It is suggested that the hindgut efferent neurons in the last abdominal ganglion are involved in: (1) innervation of the hindgut; (2) central integrative processes; (3) “en route” synaptic modification of efferent and afferent signals in the intestinal nerve; (4) neurohumoral modulation of peripheral physiological processes.
Similar content being viewed by others
References
Alexandrowicz JS (1909) Zur Kenntnis des sympathischen Nervensystem der Crustaceen. Jena Z Naturwiss 45:395–443
Bacon JP, Altman JS (1977) A silver intensification method for cobalt-filled neurons in wholemount preparations. Brain Res 138:359–363
Beltz BA, Kravitz B (1983) Mapping of serotonin-like immunoreactivity in the lobster nervous system. J Neurosci 3:585–602
Beltz BA, Kravitz B (1987) Physiological identification, morphological analysis, and development of identified serotonin-proctolin containing neurons in the lobster ventral nerve cord. J Neurosci 7:533–546
Brogan RT, Pitman RM (1981) Axonal regeneration in an identified motoneuron. J Physiol (Lond) 319:34P-35P
Bullock TH, Horridge GA (1965) Structure and Function in the Nervous System of Invertebrates. Freeman and Co, San Francisco
Davis NT (1987) Neurosecretory neurons and their projections to the serotonin neurohemal system of the cockroach Periplaneta americana (L.), and identification of mandibular and maxillary motor neurons associated with this system. J Comp Neurol 259:604–621
Elekes K, S-Rozsa K (1984) Synaptic organization of a multifunctional interneuron in the central nervous system of Helix pomatia L. Cell Tissue Res 236:677–683
Elekes K, Vehovszky A, Salanki J (1983) Ultrastructure of synaptic connections of a bimodal pacemaker giant neuron in the central nervous system of Helix pomatia L. Cell Tissue Res 239:611–620
Elekes K, Mustert R, Geffard M (1987) Serotonin-immunoreactive and dopamine-immunoreactive neurons in the terminal ganglion of the cricket, Acheta domestica. Light- and electron microscopic immunocytochemistry. Cell Tissue Res 250:167–180
Elofsson R, Kauri T, Nielsen SO, Strömberg JO (1968) Catecholamine-containing nerve fibers in the hindgut of the crayfish Astacus astacus L. Experientia 24:1159–1160
Elofsson R, Elekes K, Myhrberg H (1978) Catecholaminergic innervation of muscles in the hindgut of crustaceans. Cell Tissue Res 189:257–266
Florey E (1954) Über die Wirkung von Acetylcholin, Adrenalin, nor-Adrenalin, Faktor I und anderen Substanzen auf den isolierten Enddarm des Flußkrebses Procambarus clarkii Girard. Z Vergl Physiol 36:1–8
Florey E (1961) A new test preparation for bio-assay of Factor I and gamma-aminobutyric acid. J Physiol (Lond) 156:1–7
Hustert R, Topel U (1986) Location and major postembryonic changes of identified 5-HT-immunoreactive neurones in the terminal ganglion of a cricket. Cell Tissue Res 245:615–621
King DG (1976) Organization of crustacean neuropil. II. Distribution of synaptic contacts in identified motor neurons in lobster stomatogastric ganglion. J Neurocytol 5:239–266
King DG, Wyman RF (1980) Anatomy of the giant fibre pathway of Drosophila I. Three thoracic components of the pathway. J Neurocytol 9:753–770
Kondoh Y, Hisada M (1986) Neuroanatomy of the terminal (sixth abdominal) ganglion of the crayfish, Procambarus clarkii (Girard). Cell Tissue Res 243:273–288
Kondoh Y, Sato M, Hisada M (1987) Neuronal structure and synaptic distribution of a uropod closer motor neuron in the crayfish terminal ganglion. J Neurocytol 16:39–54
Muller KJ, McMahan UJ (1976) The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proc R Soc Lond [Biol] 194:481–499
Nässei DR, Elekes K (1985) Serotonergic terminals in the neural sheath of the blowfly nervous system: electron microscopical immunocytochemistry and 5,7-dihydroxytryptamine labelling. Neuroscience 15:293–307
Nässei DR, O'Shea M (1987) Proctolin-like immunoreactive neurons in the blowfly central nervous system. J Comp Neurol 265:437–454
Quicke DLJ, Brace RC (1979) Differential staining of cobalt- and nickel-filled neurons using rubeanic acid. J Microsc 115:161–163
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Siwiki KK, Bishop CA (1986) Mapping of proctoline-like immuno-reactivity in the nervous systems of lobster and crayfish. J Comp Neurol 243:435–453
Watson AHD, Burrows M (1981) Input and output synapses on identified motor neurones of a locust revealed by the intracellular injection of horseradish peroxidase. Cell Tissue Res 215:325–332
Watson AHD, Burrows M (1982) The ultrastructure of identified locust motor neurones and their synaptic relationships. J Comp Neurol 205:383–397
Winlow W, Laverack MS (1972 a) The control of hindgut motility in the lobster, Homarus gammarus (L.). 2. Motor output. Mar Behav Physiol 1:29–47
Winlow W, Laverack MS (1972b) The control of hindgut motility in the lobster, Homarus gammarus (L.). 3. Structure of the sixth abdominal ganglion (6 A.G.) and associated ablation and microelectrode studies. Mar Behav Physiol 1:93–121
Author information
Authors and Affiliations
Additional information
Fellow of the Alexander von Humboldt Stiftung
Rights and permissions
About this article
Cite this article
Elekes, K., Florey, E. & Cahill, M.A. Morphology and central synaptic connections of the efferent neurons innervating the crayfish hindgut. Cell Tissue Res. 254, 369–379 (1988). https://doi.org/10.1007/BF00225809
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00225809