Summary
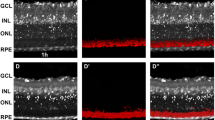
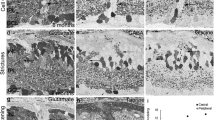
We have examined the distribution and size of nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase reactivity in adult and developing cat retinae. From late gestation E (embryonic day) 58 to adulthood, NADPH-diaphorase reactivity was detected in amacrine cells with somata located in the inner nuclear layer (INL) and ganglion cell layer (GCL) and in processes spreading in the middle strata of the inner plexiform layer (IPL). Reactivity was also present in small rounded profiles located in the outer plexiform layer (OPL) and thought to be cone pedicles. The number of NADPH-diaphorase reactive cells present in adult retinae was about 40 000; 75% of these somata were located in the GCL, the remainder in the INL. At birth, however, there was more than double this number of labelled somata (85 000), the total gradually declining to reach adult values by P (postnatal day) 25. This loss of NADPH-diaphorase reactive somata may be partly explained by natural cell death (apoptosis) or by loss of the active diaphorase from the cells. The density distributions of NADPH-diaphorase reactive cells in the INL and GCL of retinal wholemounts reached maxima in regions slightly inferior to the area centralis at all ages studied. The principal topographical difference between adult and developing retinae was that the density gradient of NADPH-diaphorase reactive cells was steeper in adults than at younger ages. During early development, the somal and dendritic field diameters of NADPH-diaphorase reactive cells at the area centralis were about the same size as those in the periphery; by adulthood, cells in the periphery were larger. The change in the somal diameter gradient apparently emerged because of a reduction in somal size of the centrally located cells. The change in the dendritic diameter gradient emerged because of a greater growth of peripheral cells as compared to central cells. We suggest that NADPH-diaphorase may have a role in the formation of synapses in the developing IPL.
Similar content being viewed by others
References
Cobcroft MD, Vaccaro TM, Mitrofanis J (1989) Distinct patterns of distribution among NADPH-diaphorase neurones in the guinea pig retina. Neurosci Lett 103:1–7
Dann JF (1989) Cholinergic amacrine cells in the developing cat retina. J Comp Neurol 289:143–155
Dann JF, Buhl EH, Piechl L (1987) Dendritic maturation in cat retinal ganglion cells: a Lucifer yellow study. Neurosci Lett 80:21–26
Dreher B, Potts RA, Ni SYK, Bennett MR (1984) The development of heterogeneities in distribution and soma sizes of rat retinal ganglion cells. In: Stone J, Dreher B, Rapaport DH (eds) Neurology and neurobiology 9. Development of visual pathways in mammals. Liss, New York, pp 39–57
Halasz P, Martin P (1984) A microcomputer based system for semi-automatic analysis of histochemical sections. R Microsc Soc Proc 19:312
Horsburgh GM, Sefton AJ (1987) Cellular degeneration and synaptogenesis in the developing retina of the rat. J Comp Neurol 263:553–566
Kowall NW, Ferante RJ, Bear MF, Richardson EP, Sofroniew MV, Cuello AC, Martin JB (1987) Neuropeptide Y, somatostatin and reduced nicotinamide adenine dinucleotide phosphate diaphorase in the human striatum: a combined immunocytochemical and enzyme histochemical study. Neuroscience 20:817–828
Kuwabara T, Weidman TA (1974) Development of the prenatal rat retina. Invest Ophthalmol 13:725–739
Maslim J, Stone J (1986) Synaptogenesis in the retina of the cat. Brain Res 373:35–48
Mastronarde DN, Thibeault MA, Dubin MW (1984) Non-uniform postnatal growth of the cat retina. J Comp Neurol 228:598–608
Mattson MP (1988) Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res Rev 13:179–212
Mitrofanis J (1989) Development of NADPH-diaphorase neurones in the rat's retina. Neurosci Lett 102:165–172
Mitrofanis J, Maslim J, Stone J (1989a) Ontogeny of catecholaminergic and cholinergic cell distributions in the cat's retina. J Comp Neurol 289:228–246
Mitrofanis J, Robinson SR, Provis JM (1989b) Somatostatinergic neurones in developing human and cat retinae. Neurosci Lett 104:209–216
Müller FH, Wässle H, Brecha NC (1988) NADPH-diaphorasepositive amacrine cells show GABA-like immunoreactivity in cat retina. Eur J Neurosci [Suppl] 1:153
Oyster CW, Takahashi ES, Cilluffo M, Brecha NC (1985) Morphology and distribution of tyrosine hydroxylase-like immunoreactive neurones in the cat retina. Proc Natl Acad Sci USA 82:6335–6339
Pearse AGE (1967) Fundamentals of functional neurochemistry. Brain Res 4:125–134
Provis JM (1979) The distribution and size of ganglion cells in the retina of the pigmented rabbit: a quantitative analysis. J Comp Neurol 185:121–138
Provis JM, Mitrofanis J (1990) NADPH-diaphorase neurones of human retinae have a uniform topographical distribution. Vis Neurosci 6:613–620
Rapaport DH, Stone J (1983) Time course of morphological differentiation of cat retinal ganglion cells. J Comp Neurol 221:42–52
Robinson SR (1987) Ontogeny of the area centralis in the cat. J Comp Neurol 255:50–67
Robinson SR (1988) Cell death in the inner and outer nuclear layers of the developing cat retina. J Comp Neurol 267:507–515
Robinson SR, DreherB, McCall MJ (1989) Non-uniform retinal expansion during the formation of the rabbit's visual streak: Implications for the ontogeny of mammalian retinal topography. Visual Neurosci 2:201–219
Sagar SM (1986) NADPH-diaphorase histochemistry in the rabbit retina. Brain Res 373:153–158
Sagar SM (1987) Somatostatin-like immunoreactive material in the rabbit retina: immunohistochemical staining using monoclonal antibodies. J Comp Neurol 266:291–299
Sagar SM, Marshall PE (1988) Somatostatin-like immunoreactivity in the human retina. Neuroscience 12:642–650
Sandell JH (1985) NADPH-diaphorase cells in the mammalian inner retina. J Comp Neurol 238:466–472
Sandell JH (1986) NADPH-diaphorase cells in the macaque striate cortex. J Comp Neurol 251:388–397
Sandell JH, Graybiel AM, Chesselet MF (1986) A new enzyme marker for striatal compartmentalization: NADPH-diaphorase activity in the caudate and putamen of the cat. J Comp Neurol 243:326–334
Scherer-Singler U, Vincent SR, Kimura H, McGeer EG (1983) Demonstration of a unique population of neurones with NADPH-diaphorase histochemistry. J Neurosci Meth 9:229–234
Scott JW, McDonald JK, Pemberton JL (1987) Short axon cells of the rat olfactory bulb display NADPH-diaphorase activity, neuropeptide Y-like immunoreactivity, and somatostatin-like immunoreactivity. J Comp Neurol 260:378–391
Stone J (1981) The wholemount handbook. Sydney, Maitland
Thomas E, Pearse AGE (1964) The solitary active cells: histochemical demonstration of damage resistent nerve cells with a TPN diaphorase reaction. Acta Neuropathol 3:238–249
Vaney DI, Young HM (1988) GABA-like immunoreactivity in NADPH-diaphorase amacrine cells of the rabbit retina. Brain Res 474:380–385
Versaux-Botteri C, Martin-Martinelli E, Nguyen-Legros J, Geffard M, Vigny A, Denoroy L (1986) Regional specialisation of the rat retina: catecholamine-containing amacrine cell characterization and distribution. J Comp Neurol 243:422–433
Vincent SR, Johansson O (1983) Striatal neurones containing both somatostatin and avian pancreatic polypeptide (APP)-like immunoreactivities and NADPH-diaphorase activity. J Comp Neurol 217:264–270
Vincent SR, Johansson O, Hökfelt T, Skirboll L, Elde RP, Terenius L, Kimmel J, Goldstein M (1983a) NADPH-diaphorase: a selective histochemical marker of striatal neurones containing both somatostatin and avian pancreatic polypeptide (APP)-like immunoreactivities. J Comp Neurol 217:252–263
Vincent SR, Satoh K, Armstrong DM, Fibiger HC (1983b) NADPH-diaphorase: a selective histochemical marker for the cholinergic neurones of the pontine reticular formation. Neurosci Lett 43:31–36
Vincent SR, Satoh K, Armstrong DM, Panula P, Vale W, Fibiger HC (1986) Neuropeptides and NADPH-diaphorase activity in the ascending cholinergic reticular system of the rat. Neuroscience 17:167–182
Wässle H, Hoon Chun M, Müller F (1987) Amacrine cells in the ganglion cell layer of the cat retina. J Comp Neurol 265:391–408
White CA, Chalupa LM, Johnson D, Brecha NC (1990) Somatostatin immunoreactivity in the adult cat retina. J Comp Neurol 293:193–150
Wong ROL, Hughes A (1987) Developing neuronal populations of the cat reginal ganglion cell layer. J Comp Neurol 262:473–495
Young HM, Vaney DI (1989) GABA-like immunoreactivity in NADPH-diaphorase amacrine cells of rabbit and rat retinae. Neurosci Lett [Suppl] 27:S 114
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vaccaro, T.M., Cobcroft, M.D., Provis, J.M. et al. NADPH-diaphorase reactivity in adult and developing cat retinae. Cell Tissue Res. 265, 371–379 (1991). https://doi.org/10.1007/BF00398085
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00398085