Summary
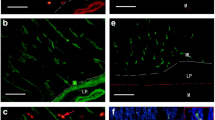
Immunocytochemical application of the antimuscarinic acetylcholine receptor antibody M35 to pancreas tissue revealed the target areas for the parasympathetic nervous system. Immunoreactivity in the endocrine pancreas was much higher than that in the exocrine part. Moreover, the endocrine cells at the periphery of the islets of Langerhans displayed the highest level of immunoreactivity. Based on these findings in the mantle of the islets, two types of islets have been distinguished: type-I islets with intensely stained mantle cells, and type-II islets with a much lower concentration of these cells. On average, type-I islets were larger (244.8 μm±6.1 SEM) than type-II islets (121.5 μm±3.8 SEM). M35-immunoreactivity was present on the majority of D cells, which were characterized by their immunoreactivity to somatostatin [of 446 D cells 356 (79.8%) were M35-immunopositive]. However, only a small proportion of the intensely stained mantle cells belonged to the D cell population. Therefore, it is concluded that the majority of the intensely stained mantle cells represent glucagon-secreting A and/or pancreatic polypeptide-secreting F cells. The intensity of M35-immunoreactivity at the periphery and central core of the islets paralleled the density of cholinergic innervation, suggesting a positive correlation between the intensity of cholinergic transmission and the number of muscarinic acetylcholine receptors at the target structures. The present study further revealed some striking parallels for the muscarinic acetylcholine receptor characteristics between the (endocrine) pancreas and the central nervous system.
Similar content being viewed by others
References
Ahren B, Taborsky GJ Jr, Porte D Jr (1986) Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 29: 827–836
André C, De Backer JP, Guillet JC, Vanderheyden P, Vauquelin G, Strosberg AD (1983) Purification of muscarinic acetylcholine receptors by affinity chromatography. EMBO J 2: 499–504
André C, Guillet JG, De Backer JP, Vanderheyden P, Hoebeke J, Strosberg AD (1984) Monoclonal antibodies against the native or denatured forms of muscarinic acetylcholine receptors. EMBO J 3: 17–21
André C, Marullo S, Guillet JG, Convents A, Lauwereys M, Kaveri S, Hoebeke J, Strosberg AD (1987) Immunochemical studies of the muscarinic acetylcholine receptor. J Recept Res 7: 89–103
Appert HE, Chiu TH, Budd GC, Leonardi AJ, Howard JM (1981) Tritium-labeled methylscopolamine binding to dispersed pancreatic acini. Cell Tissue Res 220: 673–684
Arimura A, Fishback JB (1981) Somatostatin: regulation of secretion. Prog Neuroendocrinol 33: 246–256
Bonner TI, Buckley NJ, Young AC, Brann MR (1987) Identification of a family of muscarinic acetylcholine receptor genes. Science 237: 527–532
Bonner-Weir S (1988) Morphological evidence for pancreatic polarity of B cell within the islets of Langerhans. Diabetes 37: 616–621
Bonner-Weir S (1991) Anatomy of the islet of Langerhans. In: Samois E (ed) The endocrine pancreas. Raven Press, New York, pp 15–27
Bruce G, Wainer BH, Hersh LB (1985) Immunoaffinity purification of human choline acetyltransferase: comparison of the brain and placental enzymes. J Neurochem 45: 611–620
Buckley NJ, Bonner TI, Brann MR (1988) Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci 8: 4646–4652
Coupland RE (1958) The innervation of pancreas of the rat, cat and rabbit as revealed by the cholinesterase technique. J Anat 92: 143–149
Eckenstein F, Sofroniew MV (1983) Identification of central cholinergic neurons containing both choline acetyltransferase and acetylcholinesterase and of central neurons containing only acetylcholinesterase. J Neurosci 3: 2286–2291
Fischer U, Hommel H, Ziegler M, Michael R (1972) The mechanism of insulin secretion after oral glucose administration. Diabetologia 8: 104–110
Fritschy WM, Strubbe JH, Wolters GHJ, Schilfgaarde R van (1991) Glucose tolerance and plasma insulin response to intravenous glucose infusion and test meal in rats with microencapsulated islet allografts. Diabetologia 34: 542–547
Frohman LA, Ezdinli EZ, Javid R (1967) Effect of vagotomy and vagal stimulation on insulin release. Diabetes 16: 443–448
Godfrey DA, Matschinsky FM (1975) Enzymes of the cholinergic system in islets of Langerhans. J Histochem Cytochem 23: 645–651
Grill V, Östenson C-G (1983) Muscarinic receptors in pancreatic islets of the rat. Demonstration and dependence on long-term glucose environment. Biochim Biophys Acta 756: 159–162
Hamel E, Beaudet A (1987) Ulrastructural distribution of muopioid receptors in rat neostriatum. In: Sandler M (ed) Neurotransmitter interactions in the basal ganglia. Raven Press, New York, pp 59–69
Hedreen JC, Bacon SJ, Price DL (1985) A modified histochemical technique to visualize acetylcholinesterase-containing axons. J Histochem Cytochem 33: 134–140
Henquin J-C, Nenquin M (1988) The muscarinic receptor subtype in mouse pancreatic B cells. FEBS Lett 236: 89–92
Hermansen K (1980) Secretion of somatostatin from the normal and diabetic pancreas. Studies in vitro. Diabetologia 19: 492–497
Honey RN, Weir GC (1980) Acetylcholine stimulates insulin, glucagon, and somatostatin release in the perfused chicken pancreas. Endocrinology 107: 1065–1068
Korc M, Ackerman MS, Roeske WRA (1987) Cholinergic antagonist identifies a subclass of muscarinic receptors in isolated rat pancreatic acini. J Pharmacol Exp Ther 240: 118–122
Kuhar MJ, Taylor N, Wamsley JK, Hulme EC, Birdsall NJM (1981) Muscarinic cholinergic receptor localization in brain by electron microscopic autoradiography. Brain Res 216: 1–9
Leiber D, Harbon S, Guillet JG, André C, Strosberg AD (1984) Monoclonal antibodies to purified muscarinic receptor display agonist-like activity. Proc Natl Acad Sci USA 81: 4331–4334
Levey AI, Wainer BH, Rye DB, Mufson EJ, Mesulam M-M (1984) Choline acetyltransferase-immunoreactive neurons intrinsic to rodent cortex and distinction from acetylcholinesterase-positive neurons. Neurosci 2: 341–353
Luiten PGM, Ter Horst GJ, Buijs RM, Steffens AB (1986) Autonomic innervation of the pancreas in diabetic and non-diabetic rats. A new view on intramural sympathetic structural organization. J Auton Nerv Systems 15: 33–44
Miller RE (1981) Pancreatic neuroendocrinology: peripheral neuroal mechanisms in the regulation of the islets of Langerhans. Endocr Rev 2: 471–494
Munger BL (1981) Morphological characterization of islet cell diversity. In: Cooperstein SJ, Watkins D (eds) The islets of Langerhans. Academic Press, New York, pp 3–34
Ng KH, Morrisset J, Poireir GG (1979) Muscarinic receptors of the pancreas: a correlation between displacement of 3H quinuclinidyl benzilate binding and amylase secretion. Pharmacology 18: 263–270
Östenson C-G, Grill V (1985) Glucose exerts opposite effects on muscarinic receptor binding to A and B cells of the endocrine pancreas. Endocrinology 116: 1741–1744
Östenson C-G, Grill V (1987) Evidence that hyperglycemia increases muscarinic binding in pancreatic islets of the rat. Endocrinology 121: 1705–1710
Orci L, Unger RH (1975) Functional subdivision of islets of Langerhans and possible role of D cells. Lancet II: 1243–1247
Pearse AGE (1977) The diffuse neuroendocrine system and the APUD concept: related “endocrine” peptides in brain, intestine, pituitary, placenta, and cutaneous glands. Med Biol 55: 115–125
Pearse AGE, Takor Takor T (1976) Neuroendocrine embryology and the APUD concept. Clin Endocrinol [Suppl] 5: 229s-244s
Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, Capon DJ (1987) Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J 6: 3923–3929
Pipeleers DG (1987) The biosociology of pancreatic B cells. Diabetologia 30: 277–291
Schuit FC, In't Veld PA, Pipeleers DG (1988) Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci USA 85: 3865–3869
Schusdziarra V, Harris V, Arimura A, Unger RH (1979) Evidence for a role of splanchnic somatostatin in the homeostasis of ingested nutrients. Endocrinol 104: 1705–1708
Schwartz TW, Holst JJ, Fahrenkrug J (1978) Vagal, cholinergic regulation of pancreatic polypeptide secretion. J Clin Invest 61: 781–789
Strubbe JH, Bouman PR (1978) Plasma insulin patterns in the unanesthetized rat during intracardial infusion and spontaneous ingestion of graded loads of glucose. Metabolism 27: 341–351
Strubbe JH, Steffens AB (1975) Rapid insulin release after ingestion of a meal in the unanesthetized rat. Am J Physiol 229: 1019–1022
Van der Zee EA, Matsuyama T, Strosberg AD, Traber J, Luiten PGM (1989) Demonstration of muscarinic acetylcholine receptor-like immunoreactivity in rat forebrain and upper brainstem. Histochemistry 92: 475–485
Van der Zee EA, Benoit R, Strosberg AD, Luiten PGM (1991a) Coexistence of muscarinic acetylcholine receptors and somatostatin in nonpyramidal neurons of the rat dorsal hippocampus. Brain Res Bull 26: 343–351
Van der Zee EA, Jong GI de, Strosberg AD, Luiten PGM (1991b) Parvalbumin-positive neurons in rat dorsal hippocampus contain muscarinic acetylcholine receptors. Brain Res Bull 27: 697–700
Vasudevan S, Reiländer H, Maul G, Michel H (1991) Expression and cell membrane localization of rat M3 muscarinic acetylcholine receptor produced in Sf9 insect cells using baculovirus system. FEBS Lett 283: 52–56
Verspohl EJ, Tacke R, Mutschler E, Lambrecht G (1990) Muscarinic receptor subtypes in rat pancreatic islets: binding and functional studies. Eur J Pharmacol 178: 303–311
Waelbroeck M, Camus J, Winand J, Christophe J (1987) Different antagonist binding properties of rat pancreatic and cardiac muscarinic receptors. Life Sci 41: 2235–2240
Wood SM, Polak JM, Bloom SR (1983) Neuropeptides in the control of the islets of Langerhans. In: Szabo AJ (ed) CNS regulation of carbohydrate metabolism. Academic Press, New York, pp 401–420
Zawilich WS, Zawalich KC, Rasmussen H (1989) Cholinergic agonists prime the B-cell to glucose. Endocrinology 125: 2400–2406
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van der Zee, E.A., Buwalda, B., Strubbe, J.H. et al. Immunocytochemical localization of muscarinic acetylcholine receptors in the rat endocrine pancreas. Cell Tissue Res 269, 99–106 (1992). https://doi.org/10.1007/BF00384730
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00384730