Summary
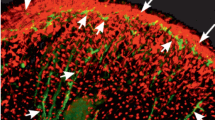
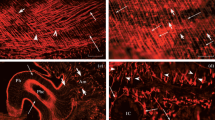
Involvement of neuropeptides in the regulation of cardiac activity in a prosobranch mollusc, Rapana thomasiana, was studied physiologically as well as immunohistochemically. A catch-relaxing peptide (CARP) showed strong inhibitory effects on the heart with a lower threshold than acetylcholine. The action of CARP was in contrast to that of another neuropeptide, FMRFamide, which has previously been shown to enhance the heart beat. Benzoquinonium blocked the effects of acetylcholine and stimulation of right cardiac nerves 1 and 3b, but not those of CARP, suggesting that the effects of nerve stimulation are mainly due to the release of acetylcholine. Immunohistochemical examinations demonstrated that FMRFamide-like and CARP-like immunoreactive neurons are distributed in the visceral ganglia. Although a neuron appeared to show weak immunoreactivity to both antisera, evidence for the coexistence of peptides in a single neuron was not exhibited. Positive immunoreactivity to FMRFamide and CARP antisera also appeared in right cardiac nerves 1 and 3. In the heart, FMRFamide- and CARP-like immunoreactive fibers were restricted to the atrium and the aortic end of the ventricle, consistent with the morphological observation of innervation. The present results suggest that FMRFamide- and CARP-like peptides are involved in regulating the heart beat.
Similar content being viewed by others
References
Buckett KJ, Peters M, Dockray GJ, Minnen J van, Benjamin PR (1990) Regulation of heartbeat in Lymnaea by motoneurons containing FMRFamide-like peptides. J Neurophysiol 63: 1426–1435
Cropper EC, Tenenbaum R, Kolks MAG, Kupfermann I, Weiss KR (1987) Myomodulin: a bioactive neuropeptide present in an identified cholinergic buccal motor neuron of Aplysia. Proc Natl Acad Sci USA 84: 5483–5486
Cropper EC, Vilim FS, Alevizos A, Tenenbaum R, Kolks MAG, Rosen S, Kupfermann I, Weiss KR (1991) Structure, bioactivity, and cellular localization of myomodulin B: a novel Aplysia peptide. Peptides 12: 683–690
Fujiwara-Sakata M, Muneoka Y, Kobayashi M (1991) Action and immunoreactivity of neuropeptides in the buccal neuromuscular system of a prosobranch mollusc, Rapana thomasiana. Cell Tissue Res 264: 57–62
Hirata T, Kubota I, Takabatake I, Kawahara A, Shimamoto N, Muneoka Y (1987) Catch-relaxing peptide isolated from Mytilus pedal ganglia. Brain Res 422: 374–376
Hirata T, Kubota I, Imada M, Muneoka Y, Kobayashi M (1989) Effects of the catch-relaxing peptide on molluscan muscles. Comp Biochem Physiol 92C: 283–288
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29: 577–580
Kanda T, Kuroki Y, Kubota I, Muneoka Y, Kobayashi M (1990) Neuropeptides isolated from the ganglia of a prosobranch mollusc, Fusinus ferrugineus. In: Yanaihara N (ed) Peptide chemistry 1989. Protein Res Foundation, Osaka, pp 39–44
Kawakami H, Kobayashi M (1984) Pharmacological approach to the analysis of regulation of molluscan heart activity. Zool Sci 1: 389–397
Kerkhoven RM, Croll RP, Minnen J van, Bogerd J, Ramkema MD, Lodder H, Boer HH (1991) Axonal mapping of the giant peptidergic neurons VD1 and RPD2 located in the CNS of the pond snail Lymnaea stagnalis, with particular reference to the innervation of the auricle of the heart. Brain Res 565: 8–16
Kobayashi M, Muneoka Y (1986) Structural requirements for FMRFamide-like activity on the heart of the prosobranch Rapana thomasiana. Comp Biochem Physiol 84C: 349–352
Kobayashi M, Muneoka Y (1989) Functions, receptors, and mechanisms of the FMRFamide-related peptides. Biol Bull, Woods Hole 177: 206–209
Kobayashi M, Muneoka Y (1990) Structure and action of molluscan neuropeptides. Zool Sci 7: 801–814
Kobayashi M, Nakamura M, Hasimoto T (1984) Innervation of the heart of a prosobranch mollusc, Rapana thomasiana. Zool Sci 1: 383–388
Kobayashi M, Muneoka Y, Fujiwara-Sakata M (1992) Involvement of neuropeptides in the regulation of heart beat of the African giant snail Achatina fulica Férrussac. In: Kuwasawa K, Hill RB, McMahon BR, Kuramoto T (eds) Comparative physiology: phylogenetic models in functional coupling of the CNS and the cardiovascular system. Karger, Basel (in press)
Lloyd PE, Kupfermann I, Weiss KR (1985) Two endogenous neuropeptides (SCPA and SCPB) produce a cAMP-mediated stimulation of cardiac activity in Aplysia. J Comp Physiol 156A: 659–667
Lloyd PE, Kupfermann I, Weiss KR (1987) Sequence of small cardioactive peptide A: A second member of a class of neuropeptides in Aplysia. Peptides 8: 179–184
Morris HR, Panico M, Karplus A, Lloyd PE, Riniker B (1982) Elucidation by FAB-MS of the structure of a new cardioactive peptide from Aplysia. Nature 300: 643–645
Painter SD, Greenberg MJ (1982) A survey of the responses of bivalve hearts to the molluscan neuropeptide FMRFamide and to 5-hydroxytryptamine. Biol Bull, Woods Hole 162: 311–332
Price DA, Greenberg MJ (1977a) Structure of a molluscan cardioexcitatory neuropeptide. Science 197: 670–671
Price DA, Greenberg MJ (1977b) Purification and characterization of a cardioexcitatory neuropeptide from the central ganglia of a bivalve molluse. Prep Biochem 7: 261–281
Price DA, Davies NW, Doble KE, Greenberg MJ (1987) The variety and distribution of the FMRFamide-related peptides in molluscs. Zool Sci 4: 395–410
Saunders SE, Bright K, Kellett E, Benjamin PR, Burke JF (1991) Neuropeptides Gly-Asp-Pro-Phe-Leu-Arg-Phe-amide (GDPFLRFamide) and Ser-Asp-Pro-Phe-Leu-Arg-Phe-amide (SDPFLRFamide) are encoded by an exon 3′ to Phe-Met-Arg-Phe-NH2 (FMRFamide) in the snail Lymnaea stagnalis. J Neurosci 11: 740–745
Vilim FS, Cropper EC, Alevizos A, Tenenbaum R, Kupfermann I, Weiss KR (1989) Structure determination and cellular localization of a novel myomodulin related octapeptide in Aplysia. Soc Neurosci Abst 15: 665
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fujiwara-Sakata, M., Kobayashi, M. Neuropeptides regulate the cardiac activity of a prosobranch mollusc, Rapana thomasiana . Cell Tissue Res 269, 241–247 (1992). https://doi.org/10.1007/BF00319615
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319615