Abstract
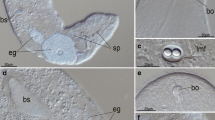
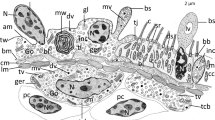
Nephrocytes are cells involved in the regulation of the composition of the hemolymph and are characterized by peripheral finger-like projections delimiting a labyrinthine channel system. We have combined structural and immunological methods to examine the spatial distribution of microtubules and microfilaments in disseminated nephrocytes of Ceratitis capitata larva; the nephrocytes are scattered among the fat-body cells, close to the salivary glands. Actin filaments are localized in two discrete peripheral domains and microtubules are mostly concentrated in the cortical region. We discuss the possibility that these cytoskeletal elements, localized in the finger-like processes of the plasma membrane, are involved in maintaining the spatial architecture of the cell periphery and in modifying the junctional complexes that represent the entrance to the labyrinthine channel system.
Similar content being viewed by others
References
Bendayan M (1985) Ultrastructural localization of cytoskeletal proteins in pancreatic secretory cells. Can J Biochem Cell Biol 63:680–690
Berridge MJ, Oschman JL (1972) Transporting epithelia. Academic Press, New York
Boer HH, Sminia T (1976) The sieve structure of slit diaphragms of podocytes and pore cells of gastropod molluscs. Cell Tissue Res 170:221–229
Boer HH, Algera NH, Lommerse AW (1973) Ultrastructure of possible sites of ultrafiltration in some gastropods, with particular reference to the auricle of the freshwater prosobranch Viviparus viviparus. Z Zellforsch 143:329–341
Bowers B (1964) Coated vesicles in the pericardial cells of the aphid Myzus persicae Sulz. Protoplasma 59:351–367
Crossley AC (1972) The ultrastructure and function of pericardial cells and other nephrocytes in an insect Calliphora erythrocephala. Tissue Cell 4:529–560
Crossley AC (1984) Nephrocytes and pericardial cells. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect phisiology, biochemistry and pharmacology, vol 3. Pergamon Press, Oxford, pp 487–515
Dallai R, Afzelius BA (1990) Microtubular diversity in insect spermatozoa: results obtained with a new fixative. J Struct Biol 103:164–179
Delhanty P, Locke M (1990) Cycles of F-actin redistribution in the dermal glands of an insect relate to secretion. J Cell Sci 96:303–311
Dustin P (1984) Microtubules. Springer, Berlin Heidelberg New York
El Shoura S (1986) Fine structure of the hemocytes and nephrocytes of Argas (Persicargas) arboreus (Ixodoidea: Argasidae). J Morphol 189:17–24
Gabe M, Cassier P, Fain-Maurel MA (1973) Donnees morphologiques sur les organes escréteurs abdominaux de Petrobius maritimus Leach (Insecte-aptérygote). Arch Anat Micr Morphol Exp 62:101–143
Giloh II, Sedat JW (1982) Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science 217:1252–1255
Henderson SC, Locke M (1992) The redeployment of F-actin in silk glands during moulting. Cell Motil Cytoskeleton 21:101–110
Humbert W (1975) Ultrastructure des nephrocytes cephaliques et abdominaux chez Tomocerus minor (Lubbock) et Lepidocyrtus curvicollis (Bourlet) (Collemboles). Int J Insect Morphol Embryol 4:307–318
Jespersen Å, Lützen J (1989) Ultrastructure of the protonephridial terminal organ in the terrestrial nemertean Geonermes pelaensis (Rhynchocoela, Enopla, Hoplonermetini). Acta Zool 70:157–162
Kelly RB (1990) Associations between microtubules and intracellular organelles. Curr Opin Cell Biol 2:105–108
Koenig JH, Ikeda K (1990) Transformational process of the endosomal compartment in nephrocytes of Drosophila melanogaster. Cell Tissue Res 262:233–244
Lane NJ (1981) Tight junctions in arthropod tissues. Int Rev Cytol 73:243–318
Lane NJ, Flores V (1988) Actin filaments are associated with the septate junctions of invertebrates. Tissue Cell 20:211–217
Lavanseau L, Lahargue J, Surleve-Bazeille JE (1981) Ultrastructure of the “Organe Rameux” of the sylkworm Bombyx mori L. (Lepidoptera: Bombycidae). Int J Insect Morphol Embryol 10:235–245
Maddrell SHP, Lane NJ, Harrison JB, Gardiner BOC (1985) DNA replication in binucleate cells of the Malpighian tubules of hemipteran insects. Chromosoma 91:201–209
Mellman I, Fuchs R, Helenius A (1986) Acidification of the endocytic and exocytic pathways. Annu Rev Biochem 55:663–700
Noirot-Timothée C, Noirot C (1980) Septate and scalariform junctions in arthropods. Int Rev Cytol 63:97–140
Nuttall GHF, Keilin D (1921) On the nephrocytes of Pediculus humanus. Parasitology 13:184–192
Puri IM (1924) Studies on the anatomy of Cimex lectularius. Parasitology 16:84–97
Rohde K, Watson N (1991) Ultrastructure of the flame bulbs and protonephridial capillaries of Microstomum sp. (Platyhelminthes, Macrostomida). Acta Zool 72:137–142
Segawa A, Yamashina S (1989) Roles of microfilaments in exocytosis: a new hypothesis. Cell Struct Funct 14:531–544
Seifert G (1976) Grenzen der deskriptiven Ultrastrukturforschung. Ent Germ 3:161–172
Seifert G, Rosenberg J (1977) Die Ultrastruktur der Nephrozyten von Peripatoides leuckarti (Onychophora: Peripatopsidae). Zoomorphologie 86:169–181
Smith VJ, Ratcliffe NA (1981) Pathological changes in the nephrocytes of the shore crab Carcinus maenas, following injection of bacteria. J Inverteb Pathol 38:113–121
Thomson JA, Gunson MM (1970) Development changes in the major inclusion bodies of polytene nuclei from larval tissues of the blowfly Calliphora stygia. Chromosoma 30:193–201
Wigglesworth VB (1970) The pericardial cells of insects: analogue of the reticuloendothelial system. J Reticuloendthelial Soc 7:208–216
Zanger K, Dannhorn DR, Seitz KA, Peters W (1991) Nephrocytes of harvestmen, Leiobunum limbatum and L. rotundum. Tissue Cell 23:7–15
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dallai, R., Riparbelli, M.G. & Callaini, G. The cytoskeleton of the ventral nephrocytes of Ceratitis capitata larva. Cell Tissue Res 275, 529–536 (1994). https://doi.org/10.1007/BF00318821
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318821