Abstract
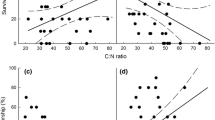
Results from laboratory feeding experiments have shown that elevated atmospheric carbon dioxide can affect interactions between plants and insect herbivores, primarily through changes in leaf nutritional quality occurring at elevated CO2. Very few data are available on insect herbivory in plant communities where insects can choose among species and positions in the canopy in which to feed. Our objectives were to determine the extent to which CO2-induced changes in plant communities and leaf nutritional quality may affect herbivory at the level of the entire canopy. We introduced equivalent populations of fourth instar Spodoptera eridania, a lepidopteran generalist, to complex model ecosystems containing seven species of moist tropical plants maintained under low mineral nutrient supply. Larvae were allowed to feed freely for 14 days, by which time they had reached the seventh instar. Prior to larval introductions, plant communities had been continuously exposed to either 340 μl CO2 l−1 or to 610 μl CO2 l−1 for 1.5 years. No major shifts in leaf nutritional quality [concentrations of N, total non-structural carbohydrates (TNC), sugar, and starch; ratios of: C/N, TNC/N, sugar/N, starch/N; leaf toughness] were observed between CO2 treatments for any of the species. Furthermore, no correlations were observed between these measures of leaf quality and leaf biomass consumption. Total leaf area and biomass of all plant communities were similar when caterpillars were introduced. However, leaf biomass of some species was slightly greater-and for other species slightly less (e.g. Cecropia peltata)-in communities exposed to elevated CO2. Larvae showed the strongest preference for C. peltata leaves, the plant species that was least abundant in all communites, and fed relatively little on plants species which were more abundant. Thus, our results indicate that leaf tissue quality, as described by these parameters, is not necessarily affected by elevated CO2 under relatively low nutrient conditions. Hence, the potential importance of CO2-induced shifts in leaf nutritional quality, as determinants of herbivory, may be overestimated for many plant communities growing on nutrient-poor sites if estimates are based on traditional laboratory feeding studies. Finally, slight shifts in the abundance of leaf tissue of various species occurring under elevated CO2 will probably not significantly affect herbivory by generalist insects. However, generalist insect herbivores appear to become more dependent on less-preferred plant species in cases where elevated CO2 results in reduced availability of leaves of a favoured plant species, and this greater dependency may eventually affect insect populations adversely.
Similar content being viewed by others
References
Arnone JA III, Körner Ch (1995) Soil and biomass carbon pools in model communities of tropical plants under elevated CO2. Oecologia 104:61–71
Arp WJ, Drake BG, Bockman WT, Curtis PS, Whigham DF (1993) Interactions between C3 and C4 salt marsh plant species during four years of exposure to elevated atmospheric CO2. Vegetatio 104/155:133–143
Bazzaz FA (1990) The response of natural ecosystems to the rising global CO2-levels. Annu Rev Ecol Syst 21:167–196
Cave T, Tolley LC, Strain BR (1981) Effect of carbon dioxide enrichment on chlorophyll content, starch content and starch grain structure in Trifolium subterraneum leaves. Physiol Plant 51:171–174
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Cipollini ML, Drake BG, Whigham DF (1993) Effects of elevated CO2 on growth and carbon/nutrient balance in the deciduous woody shrub Lindera benzoin (L.) Blume (Lauraceae). Oecologia 96:339–346
Coleman JS (1986) Leaf development and leaf stress: increased susceptibility associated with sink-source transition. Tree Physiol 2:289–299
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Crawley MJ (1983) Herbivory: the dynamics of animal-plant interactions. Blackwell, Oxford
Fajer ED (1989) The effects of enriched CO2 on plant-insect herbivore interactions: growth responses of larvae of the specialist butterfly, Junonia coenia (Lepidoptera: Nymphalidae). Oecologia 81:514–520
Fajer ED, Bowers MD, Bazzaz FA (1989) The effects of enriched carbon dioxide atmospheres on plant-insect herbivore interactions. Science 243:1198–1200
Fajer ED, Bowers MD, Bazzaz FA (1991) The effects of enriched CO2 atmospheres on the buckeye butterfly, Junonia coenia. Ecology 72:751–754
Johnson RH, Lincoln DE (1990) Sagebrush and grasshopper responses to atmospheric carbon dioxide concentration. Oecologia 84:103–110
Johnson RH, Lincoln DE (1991) Sagebrush carbon allocation patterns and grasshopper nutrition: the influence of CO2 enrichment and soil mineral limitation. Oecologia 87:127–134
Jordan CF (1985) Nutrient cycling in tropical forest ecosystems. Wiley, New York
Körner Ch, Arnone JA III (1992) Responses to elevated carbon dioxide in artificial tropical ecosystems. Science 257:1672–1675
Körner Ch, Miglietta F (1994) Long-term effects of naturally elevated CO2 on Mediterranean grassland and forest trees. Oecologia 99:343–351
Lincoln DE, Couvet D (1989) The effect of carbon supply on allocation to allelochemicals and caterpillar consumption of peppermint. Oecologia 78:112–114
Lincoln DE, Sionit N, Strain BR (1984) Growth and feeding responses of Pseudoplusia includens (Lepidoptera: Noctuidae) to host plants grown in controlled carbon dioxide atmospheres. Environ Entomol 13:1527–1530
Lincoln DE, Fajer ED, Johnson RH (1993) Plant-insect herbivore interactions in elevated CO2 environments. Trends Ecol Evol 8:64–68
Lindroth RL, Kinney KK, Platz CL (1993) Responses of deciduous trees to elevated atmospheric CO2: productivity, phytochemistry, and insect performance. Ecology 74:763–777
Mattson WJ Jr (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Nie D, Kirkham MB, Ballou LK, Lawlor DJ, Kanemasu ET (1992) Changes in prairie vegetation under elevated carbon dioxide levels and two soil moisture regimes. J Veg Sci 3:673–678
Osbrink WLA, Trumble JT, Wagner RE (1987) Host suitability of Phaseolus lunata for Trichoplusia ni (Lepidoptera: Noctuidae) in controlled carbon dioxide atmospheres. Environ Entomol 16:639–644
Owensby CE, Coyne PI, Ham JM, Auen LM, Knapp AK (1993) Biomass production in a tallgrass prairie ecosystem exposed to ambient and elevated CO2. Ecol Appl 3:644–653
Polley HW, Johnson HB, Mayeux HS (1994) Increasing CO2: comparative response of the C4 grass Schizachyrium and grassland invader Prosopis. Ecology 75:976–988
Reekie EG, Bazzaz FA (1989) Competition and patterns of resource use among seedlings of five tropical trees grown at ambient and elevated CO2. Oecologia 79:212–222
Thompson GB, Drake BG (1994) Insects and fungi on a C3 sedge and a C4 grass exposed to elevated atmospheric CO2 concentrations in open-top chambers in the field. Plant Cell Environ 17:1161–1167
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forests. Annu Rev Ecol Syst 17:137–167
Williams WE, Garbutt K, Bazzaz FA, Vitousek PM (1986) The response of plants to elevated CO2. IV. Two deciduous-forest tree communities. Oecologia 69:454–459
Wong S-C (1990) Elevated atmospheric partial pressure of CO2 and plant growth. II. Non-structural carbohydrate content in cotton plants and its effect on growth parameters. Photosyn Res 23:171–180
Wullschleger SD, Norby RJ, Hendrix DL (1992) Carbon exchange rates, chlorophyll content, and carbohydrate status of 2 forest tree species exposed to carbon dioxide enrichment. Tree Physiol 10:21–31
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arnone, J.A., Zaller, J.G., Körner, C. et al. Leaf quality and insect herbivory in model tropical plant communities after long-term exposure to elevated atmospheric CO2 . Oecologia 104, 72–78 (1995). https://doi.org/10.1007/BF00365564
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00365564