Summary
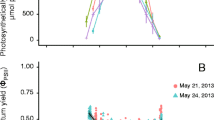
Diurnal measurements of low temperature (77K) fluorescence at 690 nm (PS II) from north, south, east, and west facing cladode surfaces of Opuntia basilaris in Death Valley, California were made on six occasions during 1985. The absolute levels of F o(instantaneous fluorescence) and F m(maximum fluorescence), as well as the ratio F v/F m(variable fluorescence, F m-F o, over maximum fluorescence), were greater in the north face relative to the other faces. Diurnal decreases in F o, F mand F v/F mwere found concomitant with increases in incident photon flux area density (PFD). F v/F mwas fairly low throughout the year, indicative of photoinhibition, but became somewhat elevated after a spring rain. In early fall the quantum yield of the south face was considerably depressed relative to that of the north face, and corresponding differences were observed in F v/F m. A decrease in PFD during growth of glasshouse plants led to an increase in chlorophyll concentration, F oand F m, but not F v/F m. Although there was some variability in the quantum yield of well watered glasshouse cladodes, a correlation was found between quantum yield and the light and CO2 saturated rate of photosynthesis. When O. basilaris was water stressed under glasshouse conditions, reductions in quantum yield, F m, and F v/F mwere observed. Reductions in F v/F malways indicated a reduced quantum yield, although the converse was not necessarily so in well watered glasshouse plants. The results of this study indicate that O. basilaris is likely to experience photoinhibition throughout much of its life in Death Valley.
Similar content being viewed by others
Abbreviations
- CAM :
-
crassulacean acid metabolism
- MPa :
-
megapascal
- PFD :
-
photon flux area density
- PS II :
-
photosystem II
- ψ :
-
vater potential
- F o :
-
instantaneous fluorescence
- F m :
-
maximum fluoescence
- F o :
-
variable fluorescence
References
Adams WW III, Nishida K, Osmond CB (1986) Quantum yields of CAM plants measured by photosynthetic O2 exchange. Plant Physiol 81:297–300
Adams WW III, Osmond CB, Sharkey TD (1987) Responses of two CAM species to different irradiances during growth and susceptibility to photoinhibition by high light. Plant Physiol (in press)
Barcikowski W, Nobel PS (1984) Water relations of cacti during desiccation: distribution of water in tissues. Bot Gaz 143:110–115
Björkman O, Demmig B (1986) Photon yield of O2 evolution and chlorophyll fluorescence at 77K among vascular plants of diverse origins. Planta (in press)
Björkman O, Powles SB (1984) Inhibition of photosynthetic reactions under water stress: interaction with light level. Planta 161:490–504
Delieu T, Walker DA (1981) Polarographic measurement of oxygen evolution by leaf discs. New Phytol 89:165–175
Delieu T, Walker DA (1983) Simultaneous measurement of oxygen evolution and chlorophyll fluorescece from leaf pieces. Plant Physiol 73:534–541
Demmig B, Björkman O (1987a) Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta (in press)
Demmig B, Björkman O (1987b) Susceptibility to photoinhibition of photosynthesis in leaves of higher plants as influenced by growth light regime. Planta (in press)
Downton WJS, Berry JA, Seemann JR (1984) Tolerance of photosynthesis to high temperature in desert plants. Plant Physiol 74:786–790
Greer D, Berry JA, Björkman O (1986) Photoinhibition of photosynthesis in intact bean leaves: Role of light and temperature and requirement for chloroplast-protein synthesis during recovery. Planta 168:253–260
Gulmon SL, Bloom AJ (1979) C3 photosynthesis and high temperature acclimation of CAM in Opuntia basilaris Engelm and Bigel. Oecologia (Berlin) 38:217–222
Hanscom Z, Ting IP (1977) Physiological responses to irrigation in Opuntia basilaris Engelm and Bigel. Bot Gaz 138:159–167
Hanscom Z, Ting IP (1978) Irrigation magnifies CAM-photosynthesis in Opuntia basilaris (Cactaceae). Oecologia (Berlin) 33:1–15
Kitajima M, Butler WL (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta 376:105–115
Ludlow MM, Björkman O (1984) Paraheliotropic leaf movement in Siratro as a protective mechanism against drought-induced damage to primary photosynthetic reactions: damage by excessive light and heat. Planta 161:505–518
Martin CE, Eades CA, Pitner RA (1986) Effects of irradiance on crassulacean acid metabolism in the epiphyte Tillandsia usneoides L. (Bromelieaceae). Plant Physiol 80:23–26
Nobel PS (1980) Interception of photosynthetically active radiation by cacti of different morphology. Oecologia (Berlin) 45:160–166
Nobel PS, Hartsock TL (1983) Relationships between photosynthetically active radiation, nocturnal acid accumulation, and CO2 uptake for a crassulacean acid metabolism plant, Opuntia ficus-indica. Plant Physiol 71:71–75
Osmond CB (1982) Carbon cycling and stability of the photosynthetic apparatus in CAM. In: Ting IP, Gibbs M (eds) Crassulacean acid metabolism. Amer Soc Plant Physiol, Rockville, Maryland, pp 112–127
Osmond CB, Winter K, Powles SB (1980) Adaptive significance of carbon dioxide cycling during photosynthesis in waterstressed plants. In: Turner NC, Kramer PJ (eds). Adaptation of plants to water and high temperature stress. John Wiley & Sons, New York, pp 139–154
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Ann Rev Plant Physiol 35:15–44
Powles SB, Björkman O (1982) Photoinhibition of photosynthesis: effect on chlorophyll fluorescence at 77K in intact leaves and in chloroplast membranes of Nerium oleander. Planta 156:97–107
Powles SB, Critchley C (1980) Effect of light intensity during growth on photoinhibition of intact attached bean leaflets. Plant Physiol 65:1181–1187
Smith SD, Didden-Zopfy B, Nobel PS (1984) High-temperature responses of North American cati. Ecology 65:643–651
Szarek SR, Ting IP (1974) Seasonal patterns of acid metabolism and gas exchange in Opuntia basilaris. Plant Physiol 54:76–81
Szarek SR, Ting IP (1975) Physiological responses to rainfall in Opuntia basilaris (Cactaceae). Amer J Bot 62:602–609
Szarek SR, Johnson HB, Ting IP (1973) Drought adaptation in Opuntia basilaris. Significance of recycling carbon through crassulacean acid metabolism. Plant Physiol 52:539–541
Vernon LP (1960) Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal Chem 32:1144–1150
Went F (1968) The mobile laboratories of the Desert Research Institute. BioScience 18:293–297
Winter K, Osmond CB, Hubick KT (1986) Crassulacean acid metabolism in the shade. Studies on an epiphytic fern, Pyrrosia longifolia, and other rainforest species from Australia. Oecologia (Berlin) 68:224–230
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Adams, W.W., Smith, S.D. & Osmond, C.B. Photoinhibition of the CAM succulent Opuntia basilaris growing in Death Valley: evidence from 77K fluorescence and quantum yield. Oecologia 71, 221–228 (1987). https://doi.org/10.1007/BF00377287
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00377287