Abstract
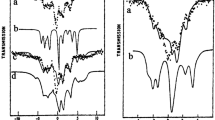
Rubredoxins contain a mononuclear iron tetrahedrally coordinated by four cysteinyl sulfurs. We have studied the wild-type protein from Clostridium pasteurianum and two mutated forms, C9S and C42S, in the oxidized and reduced states, with Mössbauer, integer-spin EPR, and magnetic circular dichroism (MCD) spectroscopies. The Mössbauer spectra of the ferric C42S and C9S mutant forms yielded zero-field splittings, D=1.2 cm−1, that are about 40% smaller than the D-value of the wild-type protein. The 57Fe hyperfine coupling constants were found to be ca. 8% larger than those of the wild-type proteins. The present study also revealed that the ferric wild-type protein has δ=0.24±0.01 mm/s at 4.2 K rather than δ=0.32 mm/s as reported in the literature. The Mössbauer spectra of both dithionite-reduced mutant proteins revealed the presence of two ferrous forms, A and B. These forms have isomer shifts δ=0.79 mm/s at 4.2 K, consistent with tetrahedral Fe2+(Cys)3(O-R) coordination. The zero-field splittings of the two forms differ substantially; we found D=−7±1 cm−1, E/D=0.09 for form A and D=+6.2±1.3 cm−1, E/D=0.15 for form B. Form A exhibits a well-defined integer-spin EPR signal; from studies at X- and Q-band we obtained g z =2.08±0.01, which is the first measured g-value for any ferrous rubredoxin. It is known from X-ray crystallographic studies that ferric C42S rubredoxin is coordinated by a serine oxygen. We achieved 75% reduction of C42S rubredoxin by irradiating an oxidized sample at 77 K with synchrotron X-rays; the radiolytic reduction produced exclusively form A, suggesting that this form represents a serine-bound Fe2+ site. Studies in different buffers in the pH 6–9 range showed that the A:B ratios, but not the spectral parameters of A and B, are buffer dependent, but no systematic variation of the ratio of the two forms with pH was observed. The presence of glycerol (30–50% v/v) was found to favor the B form. Previous absorption and circular dichroism studies of reduced wild-type rubredoxin have suggested d-d bands at 7400, 6000, and 3700 cm−1. Our low-temperature MCD measurements place the two high-energy transitions at ca. 5900 and 6300 cm−1; a third d-d transition, if present, must occur with energy lower than 3300 cm−1. The mutant proteins have d-d transitions at slightly lower energy, namely 5730, 6100 cm−1 in form A and 5350, 6380 cm−1 in form B.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 13 April 2000 / Accepted: 17 April 2000
Rights and permissions
About this article
Cite this article
Yoo, S., Meyer, J., Achim, C. et al. Mössbauer, EPR, and MCD studies of the C9S and C42S variants of Clostridium pasteurianum rubredoxin and MCD studies of the wild-type protein. JBIC 5, 475–487 (2000). https://doi.org/10.1007/s007750050008
Issue Date:
DOI: https://doi.org/10.1007/s007750050008