Abstract
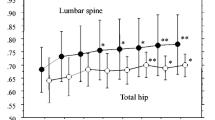
We have recently reported the results of a 24-month, double-blind, placebo-controlled study in 359 elderly osteoporotic women who were treated with daily oral alendronate (ALN) 1, 2.5, or 5 mg or placebo (PBO). We report the results of a 12-month, open-label, extension study during which 246 patients from the original study were treated with ALN 10 mg/day. Significant increases in lumbar spine bone mineral density (BMD) were observed in patients who had previously received PBO or ALN 1 and 2.5 mg/day for 24 months. Significant gains in trochanter BMD were seen in all treatment groups. Small changes were observed in femoral neck, total body, and forearm BMD during the course of this extension study. In general, the greatest increases in BMD during the open-label extension year occurred in patients who received either PBO or the lower doses of ALN during the previous 2-year blinded study. The frequencies of all categories of upper gastrointestinal adverse experiences (AEs) were less during months 25–36 (open-label extension) than during months 0–24 (original study). In conclusion, treatment with ALN 10 mg/day for 12 months in elderly women with osteoporosis who were previously treated for 24 months with PBO or ALN 1, 2.5, or 5 mg/day increased or maintained BMD of the spine, trochanter, and forearm, and was generally safe and well tolerated, especially in the upper gastrointestinal tract.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 16 June 1998 / Accepted: 1 November 1998
Rights and permissions
About this article
Cite this article
Downs, Jr., R., Bone, H., McIlwain, H. et al. An Open-Label Extension Study of Alendronate Treatment in Elderly Women with Osteoporosis. Calcif Tissue Int 64, 463–469 (1999). https://doi.org/10.1007/s002239900634
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002239900634