Summary
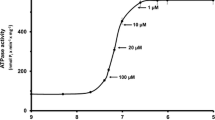
Single, slow muscle fibers fromRana temporaria were equilibrated in normal Ringer's. 95 mmol/liter K1-solution containing various concentrations of Ca2+, Ni2+, Mn2 or Mg2+ was applied, and the ensuing contractures were recorded isometrically. While peak tension (F max) was little affected, maintained tension (measured 1 min after onset of contracture) strongly depended on the concentration and species of divalent cations. Tension was maintained at its peak value in the presence of all species of divalent cations provided their concentrations were adequately increased. Dose-response curves were hyperbolic: Lineweaver-Burk plots revealed straight lines with different slopes intersecting near 1/F max, and indicating the following order of efficiency: Ni2+>Ca2+>Mn2+>>Mg2+. Hill plots for these cations resulted in straight lines with slopes near 1. Qualitatively similar relationships were obtained with contracture solutions containing D6000 (3–12 μmol/liter). However, under these conditions higher concentrations of Ca2+ or Ni2+ were required in order to fully maintain tension. After a step concentration change in the medium during contracture, the effects of Ca2+ or D600 were detectable only after a delay of 9 and 18 sec, respectively. It is concluded that divalent cations and D600 compete for the same binding site according to a 1:1 reaction. This site is presumably located inside the transverse tubular system and controls inactivation of the contractile force.
Similar content being viewed by others
References
Adrian, R.H., Chandler, W.K., Rakowski, R.F. 1976. Charge movement and mechanical repriming in skeletal muscle.J. Physiol. (London) 254:361–388
Almers, W., Fink, R., Palade, P.T. 1981. Calcium depletion in frog muscle tubules: The decline of calcium current under maintained depolarization.J. Physiol. (London) 312:177–207
Berwe, D., Gottschalk, G., Lüttgau, H.C. 1987. Effects of the calcium antagonist gallopamill (D6000) upon excitation-contraction coupling in toe muscle fibres of the frog.J. Physiol. (London) 385:693–707
Brum, G., Fitts, R., Pizarro, G., Rios, E. 1988. Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling.J. Physiol. (London) 398:475–505
Caputo, C. 1983. Pharmacological investigations of excitation-contraction coupling.In: Handbook of Physiology. Section 10. Skeletal Muscle. American Physiological Society, Bethesda, Maryland. pp. 381–415
Caputo, C., Bolaños, P. 1987. Contractile inactivation in frog skeletal muscle fibers. The effects of low calcium, tetracaine, dantrolene, D-600, and nifedipine.J. Gen. Physiol. 89:421–442
Chandler, W.K., Rakowski, R.F., Schneider, M.F. 1976. Effects of glycerol treatment and maintained deplorization on charge movement in skeletal muscle.J. Physiol. (London) 254:285–316
Eisenberg, R.S., McCarthy, R.T., Milton, R.L. 1983. Paralysis of frog skeletal muscle fibres by the calcium antogonist D-600.J. Physiol. (London) 341:495–505
Flitney, F.W. 1971. The volume of the T-system and its association with the sarcoplasmic reticulum in slow muscule fibres of the frog.J. Physiol. (London) 217:243–257
Fosset, M., Jaimovich, E., Delpont, E., Lazdunski, M. 1983. [3H]Nitrendipine receptors in skeletal muscle.J. Biol. Chem. 285:6086–6092
Frank, G.B. 1962. Utilization of bound calcium in the action of caffeine and certain multivalent cations on skeletal muscle.J. Physiol. (London) 163:254–268
Franzini-Armstrong, C. 1973. Studies on the triad. IV. Structure of the junction in frog slow fibers.J. Cell Biol. 53:120–128
Gilly, W.F., Hui, C.H. 1980. Voltage dependent charge movement in frog slow muscle fibres.J. Physiol. (London) 301:175–190
Goll, A., Ferry, D.R., Glossmann, H. 1984. Target size analysis and molecular properties of Ca2+ channels labelled with [3H]veapamil.Eur. J. Biochem. 141:177–186
Huerta, M., Muñiz, J., Stefani, E. 1986. Effects of external calcium on potassium contractures in tonic muscle fibres of the frog (Rana pipiens).J. Physiol. (London) 376:219–230
Hui, C.H., Milton, R.L., Eisenberg, R.S. 1984. Charge movement in skeletal muscle fibers paralyzed by the calcium-entry blocker D600.Proc. Natl. Acad. USA 81:2582–2585
Kotsias, B.A., Muchnik, S., Objero Paz, C.A. 1986. Co2+, low Ca2+, and verapamil reduce mechanical activity in rat skeletal muscles.Am. J. Physiol. 250:C40-C46
Krippeit-Drews, P., Schmidt, H. 1988. Maintenance of tension in slow muscle fibres ofRana temporaria: Competitive binding of divalent cations and D600.Pfluegers Arch. 412:R81
Léoty, C., Noireaud, J. 1987. Effects of external cations and calcium-channel blockers on depolarization-contraction coupling in denervated rat twitch skeletal muscles.Pfluegers Arch. 408:146–152
Lorkovié, H., Rüdel, R. 1983. Influence of divalent cations on potassium contracture duration in frog muscle fibres.Pfluegers Arch. 398:114–119
Miledi, R., Parker, I., Schalow, G. 1981. Calcium transients in normal and denervated slow muscle fibres of the frog.J. Physiol. (London) 318:191–206
Palade, P.T., Almers, W. 1985. Slow calcium and potassium currents in frog skeletal muscle: Their relationship and pharmacologic properties.Pfluegers Arch. 405:91–101
Rios, E., Brum, G. 1987. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle.Nature (London) 375:717–720
Schmidt, H. 1987. Effect of divalent cations on the K contracture of slow muscle fibers ofRana temporaria.J. Membrane Biol. 100:215–220
Schmidt, H., Siebler, M., Krippeit-Drews, P. 1988. The effect of D600 on potassium contractures of slow muscle fibres ofRana temporaria.Pfluegers Arch. 412:390–396
Schneider, M.F., Chandler, W.K. 1973. Voltage dependent charge movement in skeletal muscle: A possible step in excitation-contraction coupling.Nature (London) 242:244–246
Siebler, M., Schmidt, H. 1987. D600 prolongs inactivation of the contractile system in frog twitch fibres.Pfluegers Arch. 410:75–82
Zacharová, D., Henček, M., Radzukiewicz, T.L., Zachar, J., Nasledov, G.A. 1985. Calcium currents recorded from segments normal and denervated frog tonic muscle fibres.Gen. Physiol. Biophys. 4:641–646
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krippeit-Drews, P., Schmidt, H. Competitive action of divalent cations and D600 in frog slow muscle fibers. J. Membrain Biol. 112, 185–192 (1989). https://doi.org/10.1007/BF01871279
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871279