Abstract
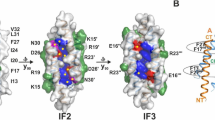
Colicin Ia is a bactericidal protein that forms voltage-dependent, ion-conducting channels, both in the inner membrane of target bacteria and in planar bilayer membranes. Its amino acid sequence is rich in charged residues, except for a hydrophobic segment of 40 residues near the carboxyl terminus. In the crystal structure of colicin Ia and related colicins, this segment forms an α-helical hairpin. The hydrophobic segment is thought to be involved in the initial association of the colicin with the membrane and in the formation of the channel, but various orientations of the hairpin with respect to the membrane have been proposed. To address this issue, we attached biotin to a residue at the tip of the hydrophobic hairpin, and then probed its location with the biotin-binding protein streptavidin, added to one side or the other of a planar bilayer. Streptavidin added to the same side as the colicin prevented channel opening. Prior addition of streptavidin to the opposite side protected channels from this effect, and also increased the rate of channel opening; it produced these effects even before the first opening of the channels. These results suggest a model of membrane association in which the colicin first binds with the hydrophobic hairpin parallel to the membrane; next the hairpin inserts in a transmembrane orientation; and finally the channel opens. We also used streptavidin binding to obtain a stable population of colicin molecules in the membrane, suitable for the quantitative study of voltage-dependent gating. The effective gating charge thus determined is pH-independent and relatively small, compared with previous results for wildtype colicin Ia.
Similar content being viewed by others
References
Cleveland, M.vB., Slatin, S., Finkelstein, A., Levinthal, C. 1983. Structure-function relationships for a voltage-dependent ion channel: properties of COOH-terminal fragments of colicin El. Proc. Natl. Acad. Sci. USA 80:3706–3710
Cramer, W.A., Heymann, J.B., Schendel, S.L., Deriy, B.N., Cohen, F.S., Elkins, P.A., Stauffacher, C.V. 1995. Structure-function of the channel-forming colicins. Annu. Rev. Biophys. Biomol. Struct. 24:611–641
Duché, D., Izard, J., González-Mañas, J.M., Parker M.W., Crest, M., Chartier, M., Baty, D. 1996. Membrane topology of the colicin A pore-forming domain analyzed by disulfide bond engineering. J. Biol. Chem. 271:15401–15406
Duché, D., Parker, M.W., González-Mañas, J.-M., Pattus, F., Baty, D. 1994. Uncoupled steps of the colicin A pore formation demonstrated by disulfide bond engineering. J. Biol. Chem. 269:6332–6339
Jakes, K.S., Abrams, C.K., Finkelstein, A., Slatin, S.L. 1990. Alteration of the pH-dependent ion selectivity of the colicin El channel by site-directed mutagenesis. J. Biol. Chem. 265:6984–6991
Jeanteur, D., Pattus, F., Timmins, P.A. 1994. Membrane-bound form of the pore-forming domain of colicin A. A neutron scattering study. J. Mol. Biol. 235:898–907
Kagawa, Y., Racker, E. 1971. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXV. Reconstitution of vesicles catalyzing 32Pi-adenosine triphosphate exchange. J. Biol. Chem. 246:5477–5487
Kienker, P., Qiu, X.-Q., Nassi, S., Slatin, S., Finkelstein, A., Jakes, K. 1996. Orientation of the hydrophobic hairpin in the colicin Ia channel. Biophys. J. 70:A140
Lakey, J.H., Baty, D., Pattus, F. 1991. Fluorescence energy transfer distance measurements using site-directed single cysteine mutants: the membrane insertion of colicin A. J. Mol. Biol. 218:639–653
Lakey, J.H., Duché, D., González-Mañas, J.-M., Baty, D., Pattus, F. 1993. Fluorescence energy transfer distance measurements: the hydrophobic helical hairpin of colicin A in the membrane bound state. J. Mol. Biol. 230:1055–1067
Lakey, J.H., Massotte, D., Heitz, F., Dasseux, J.-L., Faucon, J.-F., Parker, M.W., Pattus, F. 1991. Membrane insertion of the poreforming domain of colicin A. A spectroscopic study. Eur. J. Biochem. 196:599–607
Mankovich, J.A., Hsu, C.-H., Konisky, J. 1986. DNA and amino acid sequence analysis of structural and immunity genes of colicins Ia and Ib. J. Bacterial. 168:228–236
Massotte, D., Yamamoto, M., Scianimanico, S., Sorokine, O., van Dorsselaer, A., Nakatani, Y., Ourisson, G., Pattus, F. 1993. Structure of the membrane-bound form of the pore-forming domain of colicin A: a partial proteolysis and mass spectrometry study. Biochemistry 32:13787–13794
Mel, S.F., Falick, A.M., Burlingame, A.L., Stroud, R.M. 1993. Mapping a membrane-associated conformation of colicin Ia. Biochemistry 32:9473–9479
Montal, M. 1974. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 32:545–554
Nogueira, R.A., Varanda, W.A. 1988. Gating properties of channels formed by colicin Ia in planar lipid bilayer membranes. J. Membrane Biol. 105:143–153
Palmer, L.R., Merrill, A.R. 1994. Mapping the membrane topology of the closed state of the colicin El channel. J. Biol. Chem. 269:4187–4193
Parker, M.W., Pattus, F., Tucker, A.D., Tsernoglou, D. 1989. Structure of the membrane-pore-forming fragment of colicin A. Nature 337:93–96
Qiu, X.-Q., Jakes, K.S., Finkelstein, A., Slatin, S.L. 1994. Sitespecific biotinylation of colicin Ia. A probe for protein conformation in the membrane. J. Biol. Chem. 269:7483–7488
Qiu, X.-Q., Jakes, K.S., Kienker, P.K., Finkelstein, A., Slatin, S.L. 1996. Major transmembrane movement associated with colicin Ia channel gating. J. Gen. Physiol. 107:313–328
Shin, Y.K., Levinthal, C., Levinthal, F., Hubbell, W.L. 1993. Colicin El binding to membranes: time-resolved studies of spinlabeled mutants. Science 259:960–963
Slatin, S.L. Qiu, X.-Q., Jahes, K.S., Finkelstein, A. 1994. Identification of a translocated protein segment in a voltage-dependent channel. Nature 371:158–161
Song, H.Y., Cohen, F.S., Cramer, W.A. 1991. Membrane topography of ColEl gene products: the hydrophobic anchor of the colicin El channel is a helical hairpin. J. Bacteriol. 173:2927–2934
Stauffacher, C., Elkins, P., Cramer, W. 1996. Colicin El and the structural puzzle of the channel-forming toxins. Biophys. J. 70:A121
Wiener, M., Freymann, D., Ghosh, P., Stroud, R.M. 1997. Crystal structure in colicin Ia. Nature 385:461–464
Xu, S., Cramer, W.A., Peterson, A.A., Hermodson, M., Montecucco, C. 1988. Dynamic properties of membrane proteins: reversible insertion into membrane vesicles of a colicin El channelforming peptide. Proc. Natl. Acad. Sci. USA 85:7531–7535
Yamada, M., Ebina, Y., Miyata, T., Nakazawa, T., Nakazawa, A. 1982. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc. Natl. Acad. Sci. USA 79:2827–2831
Zakharov, S.D., Heymann, J.B., Zhang, Y.-L., Cramer, W.A. 1996. Membrane binding of the colicin El channel: activity requires an electrostatic interaction of intermediate magnitude. Biophys. J. 70:2774–2783
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kienker, P.K., Qiu, X.Q., Slatin, S.L. et al. Transmembrane insertion of the Colicin Ia hydrophobic hairpin. J. Membrane Biol. 157, 27–37 (1997). https://doi.org/10.1007/s002329900213
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s002329900213