Summary
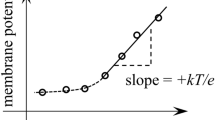
Electrical potential differences across the plasma membrane (Δψ) of the yeastPichia humboldtii were measured with microelectrodes (filled with 0.1m KCl) inserted into cells immobilized in microfunnels. The registered Δψ signals were reproducible and stable for several minutes. On attainment of stable reading for Δψ the specific membrane resistanceR sp was determined by applying square-current pulses to the preparation. Both Δψ andR sp were pH dependent and displayed equal but opposite deflection, Δψ reaching its maximal value of −88±9 mV (n=13) andR sp its minimal value of 10 kΩ·cm2 (maximal conductance) at pH 6.5. Uncouplers and the polyene antibiotic nystatin depolarized the cells, decreasing Δψ to −21±15 mV (n=10) with concomitant decrease ofR sp. Comparison of Δψ values from microelectrode measurements with those calculated from the steady-state distribution of tetraphenylphosphonium ions agreed within 10 mV under all physiological conditions tested, except at pH values above 7.0. During microelectrode insertion transient voltage signals (a few msec long) were detected by means of an oscilloscope. These voltage signals were superimposed on the stable Δψ recordings described above. These short voltage signals disappeared in uncoupled cells. The closely related Δψ values obtained by two independent methods (direct measurements with microelectrodes and calculation from steady-state distribution of a lipophilic cation) provide evidence that these values reffect the true membrane potential of intact cells.
Similar content being viewed by others
References
Bakker, R., Borst-Pauwels, G.W.F.H., Dobbelmann, J. 1986. Membrane potential in the yeastEndomyces magnusii measured by microelectrodes and TPP+ distribution.Biochim. Biophys. Acta 861:205–209
Bentrup, F.W. 1981. Botanische Elektrophysiologie.Naturwissenschaften 72:169–179
Bihler, I., Crane, R.K. 1962. Studies on the mechanism of intestinal absorption of sugars. V. The influence of several cations and anions on the active transport of sugars, in vitro, by various preparations of hamster small intestine.Biochim. Biophys. Acta 59:78–93
Boxman, A.W., Barts, P.W.J.A., Borst-Pauwels, G.W.F.H. 1982. Some characteristics of tetraphenylphosphonium uptake intoSaccharomyces cerevisae.Biochim. Biophys. Acta 686:13–18
Eraso, P., Mazon, M.J., Gancedo, J.M. 1984. Pitfalls in the measurements of membrane potential in yeast cells using tetraphenylphosphonium.Biochim. Biophys. Acta 778:516–526
Etherton, B., Keifer, D.W., Spanswick, R.M. 1977. Comparison of three methods for measuring electrical resistances in plant cell membrane.Plant Physiol. 60:864
Felle, H., Porter, J.S., Slayman, C.L., Kaback, H.R. 1980. Quantitative measurements of membrane potential inEscherichia coli.Biochemistry 19:3585–3590
Gimmler, H., Greenway, H. 1983. Tetraphenylphosphonium (TPP+) is not suitable for the assessment of electrical potential inChlorella emersonii.Plant Cell Environ. 6:739–744
Hauer, R., Höfer, M. 1978. Evidence for interactions between the energy-dependent transport of sugars and the membrane potential in the yeastRhodotorula gracilis. (Rhodosporidium toruloides).J. Membrane Biol. 43:335–349
Hedenström, M. von, Höfer, M. 1979. The effect of nystatin on active transport inRhodotorula glutinis (gracilis) is restricted to the plasma membrane.Biochim. Biophys. Acta 555:169–174
Higinbotham, N. 1973. Electropotentials of plant cells.Annu. Rev. Plant Physiol. 24:25–46
Höfer, M., Künemund, A. 1984. Tetraphenylphosphonium is a true indicator of negative plasma-membrane potential in the yeastRhodotorula glutinis.Biochem. J. 225:815–819
Höfer, M., Novacky, A. 1986. Measurements of plasma membrane potentials of yeast cells with microelectrodes.Biochim. Biophys. Acta 862:372–378
Komor, E., Tanner, W. 1974. The hexose proton symport system ofChlorella vulgaris. Specificity, stoichiometry, energetics of sugar induced proton uptake.Eur. J. Biochem. 70:197–204
Kovac, L., Varecka, L. 1981. Membrane potentials in respiring and respiration-deficient yeasts monitored by a fluorescent dye.Biochim. Biophys. Acta 637:209–216
Lammert, T., Prasad, R., Höfer, M. 1987. Relevance of cation exchange capacity of cell wall in detecting H+-symport in yeasts.Biochem. Int. 15:753–759
Lassen, U.V., Rasmussen, B.E. 1978. Use of microelectrodes for measurement of membrane potentials.In: Membrane Transport in Biology: Concepts and Models. D.C. Tosteson, editor. pp. 169–203. Springer, Berlin
Li, Z.S., Delroit, S. 1987. Osmotic dependence of the transmembrane potential difference of broadbean mesocarp cells.Plant Physiol. 84:895–899
Novacky, A., Karr, A.L., Sambeck, J.W., van 1976. Using electrophysiology of study plant disease development.Bio-Science 26:499–504
Pantoja, O., Willmer, C.M. 1986. Pressure effects on membrane potentials of mesophyll protoplasts and epidermal cell protoplasts ofCommelina communis L.J. Exp. Bot. 37:315–320
Pena, A., Uribe, S., Pardo, J.P., Barbolia, M. 1984. The use of a cyanine dye in measuring membrane potential in yeast.Arch. Biochem. Biophys. 231:217–225
Prasad, R., Höfer, M. 1986. Tetraphenylphosphonium is an indicator of negative membrane potential inCandida albicans.Biochim. Biophys. Acta 861:377–380
Robinson, J.D., Flashner, M.S. 1979. The (Na++K+)-activated ATPase. Enzymatic and transport properties.Biochim. Biophys. Acta 549:145–179
Rottenberg, H. 1978. The measurement of membrane potential and pH in cells, organelles and vesicles.Methods Enzymol. 55:547–586
Schmidt, R.F. 1971. Neurophysiologie. Springer, Berlin, Heidelberg, New York
Seaston, A., Carr, G., Eddy, A. 1976. The concentration of glycine by preparations of the yeastSaccharomyces carlsbergensis depleted of adenosin triphosphate.Biochem. J. 154:669–676
Slayman, C.L. 1965. Electrical properties ofNeurospora crassa: Effects of external cations on the intracellular potential.J. Gen. Physiol. 49:69–92
Slayman, C.L., Gradmann, D. 1975. Electrogenic proton transport in the plasma membrane ofNeurospora crassa.Biophys. J. 15:968–971
Slayman, C.L., Slayman, C.W. 1974. Depolarization of the plasmamembrane ofNeurospora crassa during active transport of glucose: Evidence for a proton dependent cotransport system.Proc. Natl. Acad. Sci. USA 71:1935–1939
Vacata, V., Kotyk, A., Sigler, K. 1981. Membrane potentials in yeast cells measured by direct and indirect methods.Biochim. Biophys. Acta 643:265–268
West, I.C., Mitchell, P. 1973. Stoichiometry of lactose proton symport across the plasma membrane ofEscherichia coli.J. Bioenerg. 3:445–462
Wolk, U., Höfer, M. 1987. Interactions between cyanine dyes and yeast cells. Do cyanine dyes act as membrane potential sensitive probes?Biochem. Int. 14:501–509
Zimmermann, U., Steudle, E. 1974. The pressure-dependence of the hydraulic conductivity, the membrane resistance and membrane potential during turgor pressure regulation inValonia utricularis.J. Membrane Biol. 16:331–352
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lichtenberg, H.C., Giebeler, H. & Höfer, M. Measurements of electrical potential differences across yeast plasma membranes with microelectrodes are consistent with values from steady-state distribution of tetraphenylphosphonium inPichia humboldtii . J. Membrain Biol. 103, 255–261 (1988). https://doi.org/10.1007/BF01993985
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01993985