Summary
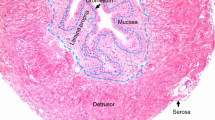
The epithelium of the urinary bladder ofBufo marinus is composed of 5 cell types, i.e., granular (Gr), mitochondria-rich (MR) and goblet (G) cells which face the urinary lumen, microfilament-rich (MFR) and undifferentiated cells (Un) located basally. The epithelium was dissociated by collagenase and EGTA treatment. Fractionation of dispersed cells by isopycnic centrifugation on dense serum albumin solutions yielded 4 fractions: (i) a very light fraction (\(\bar \rho \simeq 1.025\)) enriched in MR and MFR cells; (ii) a light fraction (\(\bar \rho \simeq 1.045\)) enriched in vacuolated Gr cells; (iii) a heavy fraction (\(\bar \rho \simeq 1.065\)) composed essentially of aggregated Gr cells, and (iv) a pellet (\(\bar \rho \simeq 1.085\)) enriched in G and undifferentiated cells. Recoveries were based on cell counts and DNA measurements. DNA content per cell was 13.2 pg±0.9 (n=37). From 1 g fresh tissue, 62±5×106 (n=10) cells were recovered before isopycnic centrifugation of which about 70% excluded Trypan blue. After centrifugation, 90 to 95% of the cells excluded the vital dye and ∼39×106 cells were recovered from the gradient. Cell metabolism in each fraction was estimated by oxygen consumption measurements in absence or presence of ouabain, acetazolamide, and dinitrophenol. The consumption was threefold higher in the very light and light fractions when compared to the heavy and pellet fractions. Ouabain sensitive oxygen consumption (QO2) represented 12 to 35% of the total O2 consumption depending on the cell fraction, and acetazolamide sensitive QO2 varied from −0.8% in the heavy fractions to 20% in the lighter fractions. DNP increased QO2 in all fractions by 20 to 50%. Finally, the cells were able to reaggregate and form junctional complexes upon addition of calcium to the medium.
Similar content being viewed by others
References
Amsterdam, A., Jamieson, J.D. 1974. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristic of separated cells. J. Cell Biol.63:1037
Bachmann, K. 1970. Specific nuclear DNA amounts in toads of the genusBufo.Chromosoma 29:365
Caplan, S.R., Essig, A. 1977. A thermodynamic treatment of active sodium transport.In: Current Topics in Membranes and Transport. F. Bronner and A. Kleinzeller, editors. p. 145. Academic Press, New York
Choi, J.K. 1963. The fine structure of the urinary bladder of the toad,Bufo marinus. J. Cell Biol. 16:53
Conger, A.D., Clinton, J.H. 1973. Nuclear volumes, DNA contents and radiosensitivity in whole body irradiated amphibians.Radiat. Res. 54:69
Edelman, I.S. 1975. Mechanism of action of steroid hormones.J. Steroid Biochem. 6:147
Gatzy, J.T., Berndt, O. 1968. Isolated epithelial cells of the toad bladder. Their preparation, oxygen consumption, and electrolyte content.J. Gen. Physiol. 51:770
Gunther, G.R., Schultz, G.S., Hull, B.E., Alicea, H.A., Jamieson, J.D. 1977. Functional pancreatic acini prepared using collagenase with low clostripain activity.J. Cell Biol. 75:368a
Handler, J.S., Orloff. 1973. The mechanism of action of antidiuretic hormone.In: Handbook of Physiology. Sect. 8, Chap. 24, pp. 791–814. Am. Physiol. Soc. Washington, D.C.
Handler, J.S., Preston, A.S. 1976. Study of enzymes regulating vasopressin-stimulated cyclic AMP metabolism in separated mitochondria-rich and granular epithelial cells of toad urinary bladder.J. Membrane Biol. 26:43
Hays, R.M., Singer, B., Malamed, S. 1965. The effect of calcium withdrawal on the structure and function of the toad bladder.J. Cell Biol. 25:195
Kissane, J.M., Robins, E. 1958. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system.J. Biol. Chem. 233:184
Kraehenbuhl, J.P. 1977. Dispersed mammary gland epithelial cells. I. Isolation and separation procedures.J. Cell Biol. 72:406
Kraehenbuhl, J.P., Pfeiffer, J., Rossier, M., Rossier, B.C. 1979. Microfilament-rich cells in the toad bladder epithelium.J. Membrane Biol. 48:167
Leaf, A., Anderson, J., Page, L.B. 1958. Active sodium transport by isolated toad bladder.J. Gen. Physiol. 41:657
Leaf, A., Hays, R.M. 1962. Permeability of the isolated toad bladder to solutes and its modification by vasopressin.J. Gen. Physiol. 45:921
Lipton, P. 1972. Effect of changes in osmolarity on sodium transport across isolated toad bladder.Am. J. Physiol. 222:821
Lipton, P., Edelman, I.S. 1971. Effects of aldosterone and vasopressin on electrolytes of toad bladder epithelial cells.Am. J. Physiol. 221:733
Lowry, O.H., Rosebrough, N.J., Fahr, A.L., Randall, R.J. 1951. Protein measurement with the Folin phenol reagent.J. Biol. Chem. 193:265
Ludens, J.H., Fanestil, D.D. 1972. Acidification of urine by the isolated urinary bladder of the Columbian toad.Am. J. Physiol. 226:1321
McKnight, A.D.C., DiBona, D.R., Leaf, A., Civan, M.M. 1971. Measurement of the composition of epithelial cells from the toad urinary bladder.J. Membrane Biol. 6:108
Peachey, L.D., Rasmussen, H. 1961. Structure of the toad's urinary bladder correlated to its physiology,J. Biophys. Biochem. Cytol. 10:529
Reisner, Y., Ravid, A., Sharon, N. 1976. Use of soybean agglutinin for the separation of mouse B and T lymphocytes.Biochem. Biophys. Res. Commun. 72:1585
Romrell, L.J., Coppe, M.R., Munro, D.R., Ito, S. 1975. Isolation and separation of highly enriched fractions of viable mouse gastric parietal cells by velocity sedimentation.J. Cell Biol. 65:428
Scott, W.N., Sapirstein, V.S. 1974. Partition of tissue functions in epithelia: Localization of enzymes in “mitochondria-rich” cells of toad urinary bladder.Science 184:797
Steinman, R.M., Cohn, Z.A. 1974. Identification of a novel cell type in peripheral lymphoid organs of mice.J. Exp. Med. 139:380
Voûte, C.L., Mollgard, K., Ussing, H.H. 1975. Quantitative relationship between active sodium transport, expansion of endoplasmic reticulum and specialized (“scalloped sacs”) in the outermost living cell layer of the frog skin epithelium.J. Membrane Biol. 21:273
Voûte, C.L., Thummel, J., Brenner, M. 1975. Aldosterone effect in the epithelium of the frog skin — a new story about an old enzyme.J. Steroid Biochem. 6:1175
Walser, M., Butler, S.E., Hammond, V. 1969. Reversible stimulation of Na+ transport in the toad bladder by stretch.J. Clin. Invest. 48:1714
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rossier, M., Rossier, B.C., Pfeiffer, J. et al. Isolation and separation of toad bladder epithelial cells. J. Membrain Biol. 48, 141–166 (1979). https://doi.org/10.1007/BF01872856
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01872856