Summary
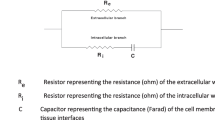
The basic electrical properties of an isolated rat hepatocyte couplet (IRHC) system have been analyzed using classical techniques of epithelial electrophysiology, including measurement of electric potentials, resistances and intracellular ion activities. Applications of these techniques are discussed with respect to their limitations in small isolated cells. Mean intracellular and intracanalicular membrane potentials ranged from −23.7 to −46.7 and −4.3 to −5.9 mV, respectively. Membrane resistances were determined using an equivalent circuit analysis modified according to the geometry of the IRHC system. Resistances of the sinusoidal (basolateral) and canalicular (luminal) cell membranes and tight junctions averaged 0.15 and 0.78 GΩ and 25mΩ, respectively. The cells are electrically coupled via low resistance intercellular communications (∼58 MΩ). Intracellular ion activities for Na+, K+ and Cl− averaged 12.2, 88.1 and 17.7 mmol/liter, respectively. The basolateral membrane potential reveals a permeability sequence ofP K>P Cl>P Na. The luminal potential showed minimal dependence on changes in transjunctional ion gradients, indicating a poor ion selectivity of the paracellular pathway. The electrogenic (Na+−K)-ATPase contributes little to the luminal and cellular negative electric potential. Therefore, the luminal potential probably results from the secretion of impermeant ions and a Donnan distribution of permeant ions, a mechanism which provides the osmotic driving force for bile formation. By providing the unique opportunity to measure luminal potentials, this isolated hepatocyte system permits study of secretory mechanisms for the first time in a mammalian gland using electrophysiologic techniques.
Similar content being viewed by others
References
Anwer, M.S., Hegner, D. 1983. Role of inorganic electrolytes in bile acid-independent canalicular bile formation.Am. J. Physiol. 244:116–124
Beck, F., Dörge, A., Mason, J., Rick, R., Thurau, K. 1982. Element concentrations of renal and hepatic cells under potassium depletion.Kidney Int. 22:250–256
Blant, M.R., Slayman, C.L. 1983. KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell.J. Membrane Biol. 72:223–234
Blitzer, B.L., Boyer, J.L. 1978. Cytochemical localization of Na+, K+-ATPase in the rat hepatocyte.J. Clin. Invest. 62:1104–1108
Blitzer, B.L., Ratoosh, S.L., Donovan, C.B., Boyer, J.L. 1982. Effects of inhibitors of Na+-coupled ion transport on bile acid uptake by isolated rat hepatocytes.Am. J. Physiol. 243:G48-G53
Boulpaep, E.L., Sackin, H. 1979. Equivalent electrical circuit analysis and rheogenic pumps in epithelia.Fed. Proc. 38:2030–2036
Boyer, J.L., Ng, O.-C., Gautam, A. 1985. Formation of canalicular spaces in isolated rat hepatocyte couplets.Trans. Assoc. Am. Phys. 98:21–29
Capiod, T., Ogden, D. 1985. Noise analysis of α-adrenergic activated K-conductance in isolated guinea pig hepatocytes.J. Physiol. (London) 369:107P
Claret, M. 1979. Transport of ions in liver cells.In: Membrane Transport in Biology. G. Giebisch, D.C. Tosteson, and H.H. Ussing, editors. Vol. 4, pp. 899–920. Springer-Verlag, Berlin-Heidelberg-New York
Claret, M., Coraboeuf, E., Favier, M.P. 1970. Effect of ion concentration changes on membrane potential of perfused rat liver.Arch. Int. Physiol. Biochim. 78:531–545
Claret, M., Mazet, J.L. 1972. Ionic fluxes and permeabilities of cell membranes in rat liver.J. Physiol. (London) 223:279–295
Claude, P. 1978. Morphological factors influencing transepithelial permeability: A model for the resistance of the zonula occludens.J. Membrane Biol. 39:219–232
Cohen, R.D., Henderson, R.M., Iles, R.A., Smith, J.A. 1982. Metabolic inter-relationships of intracellular pH measured by double-barrelled micro-electrodes in perfused rat liver.J. Physiol. (London) 330:69–80
Douglas, W.W., Taraskevich, P.S. 1985. The electrophysiology of the adenohypophyseal cells.In: Electrophysiology of the Secretory Cell. A.M. Poisner and J.M. Trifaro, editors. pp. 63–92. Elsevier, Amsterdam
Fitz, J.G., Scharschmidt, B.F. 1985. Regulation of transmembrane electrical potential (E m ) of rat hepatocytesin situ. (Abstr.) Hepatology 5:1011
Gautam, A., Scaramuzza, D., Boyer, J.L. 1986. Quantitative assessment of primary canalicular secretion in isolated rat hepatocyte couplets (IRHC) by optical planimetry. (Abstr.)Gastroenterology 90:1727
Graf, J. 1976. Sodium pumping and bile secretion.In: The Liver: Quantitative Agents of Structure and Function. R. Presig, J. Bircher and G. Paumgartner, editors. pp. 370–385. Edito Cantor, Aulendorf, W. Germany
Graf, J. 1983. Canalicular bile salt independent bile formation: Concepts and clues for electrolyte transport in rat liver.Am. J. Physiol. 244:G233-G246
Graf, J., Gautam, A., Boyer, J.L. 1984. Isolated rat hepatocyte couplets: A primary secretory unit for electrophysiologic studies of bile secretory function.Proc. Natl. Acad. Sci. USA 81:6516–6520
Graf, J., Giebisch, G. 1979. Intracellular sodium activity and sodium transport inNecturus gallbladder epithelium.J. Membrane Biol. 47:327–355
Graf, J., Peterlik, M. 1975. Mechanisms of transport of inorganic ions into bile.In: The Hepatobiliary System-Fundamental and Pathological Mechanisms. W. Taylor, editor. pp. 43–58. Plenum, New York
Graf, J., Petersen, O.H. 1974. Electrogenic sodium pump in mouse liver parenchymal cells.Proc. R. Soc. London B 187:363–367
Graf, J., Petersen, O.H. 1978. Cell membrane potential and resistance in liver.J. Physiol. (London) 284:105–126
Henderson, R.M., Graf, J., Boyer, J.L. 1987. Na−H exchange regulates intracellular pH in isolated rat hepatocyte couplets.Am. J. Physiol. 252:G109-G113
Hodgkin, A.L., Katz, B. 1949. The effect of sodium ions on the electrical activity of the giant axon of the squid.J. Physiol. (London) 108:37–77
Hosoi, S., Slayman, C.L. 1985. Membrane voltage, resistance, and channel switching in isolated mouse fibroblasts (L cells): A patch electrode analysis.J. Physiol. (London) 367:267–290
Kernan, R.P., MacDermott, M. 1980. Measurement of potassium and chloride activities in liver cells and in extensor digitorum longus muscle fibres of anaesthetised rats in situ.(abstr.) Proc. Int. Union. Physiol. Sci. 14:510
Klos, C., Paumgartner, G., Reichen, J. 1979. Cation anion gap and choleretic properties of rat bile.Am. J. Physiol. 236:E434-E441
Latham, P.S., Kashgarian, M. 1979. The ultrastructural localization of transport ATPase in the rat liver at non-bile canalicular plasma membrane.Gastroenterology 76:988–996
Layden, T.J., Elias, E., Boyer, J.L. 1978. Bile formation in the rat.J. Clin. Invest. 62:1375–1385
Loewenstein, W.R. 1979. Functional intercellular communication and the control of growth.Biochim. Biophys. Acta 560:1–65
Meyer, D.J., Yancey, S.B., Revel, J.P. 1981. Intercellular communication in normal and regenerating rat liver: A quantitative analysis.J. Cell Biol. 91:505–523
Oberleithner, H., Schmidt, B., Dietl, P. 1986. Fusion of renal epithelial cells: A novel model for studying cellular mechanisms of ion transport.Proc. Natl. Acad. Sci. USA 83:3547–3551
Penn, R.D. 1966. Ionic communications between liver cells.J. Cell Biol. 29:171–174
Reverdin, E., Weingart, R. 1986. Electrical coupling studied in cell pairs isolated from adult rat liver.J. Physiol. (London) 372:46P
Sackin, H., Boulpaep, E.L. 1983. Rheogenic transport in the renal proximal tubule.J. Gen. Physiol. 82:819–851
Scharschmidt, B.F., Stephens, J.E. 1981. Transport of sodium, chloride, and taurocholate by cultured rat hepatocytes.Proc. Natl. Acad. Sci USA 78:986–990
Seglen, P.O. 1976. Preparation of isolated rat liver cells.Methods Cell. Biol. 13:29–83
Spray, D.C., Ginzberg, R.D., Morales, E.A., Gatmaitan, Z., Arias, I.M. 1986. Electrophysiological properties of gap junctions between dissociated pairs of rat hepatocytes.J. Cell Biol. 103:135–144
Thomas, R.C., Cohen, C.J. 1981. A liquid ion-exchanger alternative to KCl for filling intracellular reference microelectrodes.Pfluegers Arch. 390:96–98
Trifaro, J.M., Poisner, A.M. 1985. Electrophysiological properties of secretory cells: On an overview.In: The Electrophysiology of the Secretory Cell. A.M. Poisner and J.M. Trifaro, editors. pp. 269–302. Elsevier, Amsterdam
Van Dyke, R.W., Scharschmidt, B.F. 1983. (Na, K)-ATPase mediated cation pumping in cultured rat hepatocytes.J. Biol. Chem. 258:12,912–12,919
Van Dyke, R.W., Stephens, J.E., Scharschmidt, B.F. 1982. Effect of ion substitution on bile formation by the isolated perfused rat liver.J. Clin. Invest. 70:505–517
Wanson, J.-C., Drochmans, P., Mosselmans, R., Rouveaux, M.-F. 1977. Adult rat hepatocytes in primary monolayer culture.J. Cell Biol. 74:858–877
Weibel, E.R., Staubli, W., Gnagi, H.R., Hess, F.A. 1969. Correlated morphometric and biochemical studies on the rat liver.J. Cell Biol. 42:68–91
Wondergem, R., Harder, D.R., 1980. Membrane potential measurements during rat liver regeneration.J. Cell. Physiol. 102:193–197
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Graf, J., Henderson, R.M., Krumpholz, B. et al. Cell membrane and transepithelial voltages and resistances in isolated rat hepatocyte couplets. J. Membrain Biol. 95, 241–254 (1987). https://doi.org/10.1007/BF01869486
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01869486