Abstract
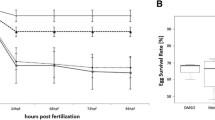
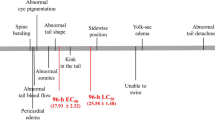
In addition to survival and hatching parameters, cytological alterations in liver and kidney of 4- and 6-d old zebrafish larvae (Brachydanio rerio) following single microinjection of fertilized eggs at the germ-ring stage with 5, 12.5, and 25 ng 4-chloroaniline/egg were investigated by means of electron microscopy. Whereas survival remained unaffected, microinjection with 4-chloroaniline disturbed hatching of larvae. Hatching was delayed by microinjection of 12.5 ng 4-chloroaniline/egg and above when compared to controls. Cytological investigations revealed ultrastructural changes in both liver and kidney in a dose- and time-dependent fashion. In the liver, major cytopathological changes included fenestration, fragmentation, and vesiculation of the rough endoplasmic reticulum, proliferation of atypical mitochondria, and atypical lysosomes. Furthermore, myelin whorls, lipid inclusions, and cholesterol crystals were increased, whereas glycogen stores were reduced. Renal tubular cells displayed altered brush borders, proliferation of nucleoli, atypical mitochondria, fenestrated, fragmented, and vesiculated RER cisternae, as well as giant lysosomes. Most of these effects indicate cellular dysfunction (e.g., disturbance of lipid metabolism in the liver), whereas others illustrate general cellular stress-responses to chemical aggression. Comparisons of results with those of previous studies based on conventional fish exposure prove the suitability and sensitivity of microinjection bioassays with zebrafish eggs as an alternative to conventional early life-stage tests.
Similar content being viewed by others
References
Babich H, Borenfreund E (1991) Cytotoxicity and genotoxicity assays with cultured fish cells: A review. Toxic Vitro 5:91–100
Baksi SM, Frazier JM (1990) Review: Isolated fish hepatocytes-Model systems for toxicology research. Aquat Toxicol 16:229–256
Benedeczky I, Nemcsok J, Halasy K (1986) Electron microscopic analysis of the cytopathological effect of pesticides in the liver, kidney, and gill tissues of carp. Acta Biol Szeged 32:69–91
Biagianti S, Bruslé J (1984) Analyse ultrastructurale des altérations hépatiques induites chez des poissons marins (Mugilidés) par un herbicide: l'atrazine (s-triazine). Biol Cell 51, 40A
Black JJ, Maccubbin AE, Schiffert M (1985) A reliable, efficient, microinjection apparatus and methodology for the in vivo exposure of rainbow trout and salmon embryos to chemical carcinogens. J Nat Cancer Inst 75:1123–1128
Braunbeck T (1993) Cytological alterations in isolated hepatocytes from rainbow trout (Oncorhynchus mykiss) exposed to 4-chloroaniline. Aquat Toxicol 25:83–110
— (1994) Detection of environmentally relevant pesticide concentrations using cytological parameters: Pesticide specificity in the reaction of rainbow trout liver? In: Müller R, Lloyd R (eds) Sublethal and chronic effects of pollutants on freshwater fish. Blackwell Scientific, Oxford, pp 15–29
Braunbeck T, Völkl A (1991) Induction of biotransformation in the liver of eel (Anguilla anguilla L.) by sublethal exposure to dinitro-o-cresol: An ultrastructural and biochemical study. Ecotox Environ Safety 21:109–127
— — (1993) Toxicant-induced cytological alterations in fish liver as biomarkers of environmental pollution? A case-study on hepatocellular effects of dinitro-o-cresol in golden ide (Leuciscus idus melanotus). In: Braunbeck T, Hanke W, Segner H (eds) Fish in ecotoxicology and ecophysiology. VCH, Weinheim, pp 55–80
Braunbeck T, Storch V, Nagel R (1989) Sex-specific reaction of liver ultrastructure in zebra fish (Brachydanio rerio) after prolonged sublethal exposure to 4-nitrophenol. Aquat Toxicol 14:185–202
Braunbeck T, Storch V, Bresch H (1990a) Species-specific reaction of liver ultrastructure of zebra fish (Brachydanio rerio) and trout (Salmo gairdneri) after prolonged exposure to 4-chloroaniline. Arch Environ Contam Toxicol 19:405–418
Braunbeck T, Görge G, Storch V, Nagel R (1990bs) Hepatic steatosis in zebra fish (Brachydanio rerio) induced by long-term exposure to hexachlorocyclohexane. Ecotox Environ Safety 19:355–374
Braunbeck T, Burkhardt-Holm P, Görge G, Nagel R, Negele RD, Storch V (1992a) Regenbogen forelle und Zabrabärbling, zwei Modelle für verlängerte Toxizitätstests: Relative Empfindlichkeit, Art- und Organspezifität in der cytopathologischen Reaktion von Leber und Darm auf Atrazin. Schriftenr Ver Wasser-, Boden-, Lufthygiene 89:109–145
Braunbeck T, Teh SJ, Lester SM, Hinton DE (1992b) Ultrastructural alterations in hepatocytes of medaka (Oryzias latipes) exposed to diethylnitrosamine. Toxicol Pathol 20:179–196
Bresch H, Beck H, Ehlermann D, Schlaszus H, Urbanek M (1990) A long-term toxicity test comprising reproduction and growth of zebrafish with 4-chloroaniline. Arch Environ Contam Toxicol 19:419–427
Burkhardt-Holm P, Oulmi Y, Schroeder A, Braunbeck T (1996) Toxicity of 4-chloroaniline in early life-stages of zebrafish (Brachydanio rerio): I. Cytopathology of liver and gills after conventional exposure to water-borne 4-chloroaniline. Arch Environ Contam Toxicol (submitted)
Dave G, Xiu R (1991) Toxicity of mercury, copper, nickel, lead, and cobalt to embryos and larvae of zebrafish Brachydanio rerio. Arch Environ Contam Toxicol 21:126–134
de Monte Westerfield (1993) The zebrafish book: A guide for the laboratory use of zebrafish (Brachydanio rerio). University of Oregon Press
Denucé JM (1985) How embryos escape from their envelopes: A new look at the (phylogenetically) old problem of hatching. Meded Kon Acad Wet Belgie 46:1–30
Fent K, Meier W (1994) Effects of triphenyltin on fish early life stages. Arch Environ Contam Toxicol 27:224–231
— — (1992) Tributyltin-induced effects on early life stages of minnows, Phoxinus phoxinus. Arch Environ Contam Toxicol 22:428–438
Fischer-Scherl T, Veeser A, Hoffman RW, Kuhnhauser C, Negele RD, Ewringmann T (1991) Morphological effects of acute and chronic atrazine exposure in rainbow trout (Oncorhynchus mykiss). Arch Environ Contam Toxicol 20:454–461
Görge G, Nagel R (1990) Toxicity of lindane, atrazine, and deltamethrin to early life stages of zebrafish (Brachydanio rerio). Ecotox Environ Safety 20:246–255
Grizzle JM, Putnam MR, Fournie JW, Couch JA (1988) Microinjection of chemical carcinogens into small fish embryos: Exocrine pancreatic neoplasm in Fundulus grandis exposed to N-methyl-N′-nitro-N-nitrosoguanidine. Dis Aquat Org 5:101–105
Hacking MA, Budd J, Hodson K (1978) The ultrastructure of the liver rainbow trout: Normal structure and modifications after chronic administration of a polychlorinated biphenyl Aroclor 1254. Can J Zool 56:477–491
Hentschel H, Elger M (1987) The distal nephron in the kidney of fishes. Adv Anat Embryol Cell Biol 106:1–151
Karnovsky MJ (1971) Use of ferrocyanide-reduced osmium tetroxide in electron microscopy. J Cell Biol 51:Abstr 284
Klaunig JE, Lipsky MM, Trump BF, Hinton DE (1979) Biochemical and ultrastructural changes in teleost liver following subacute exposure to PCB. J Environ Pathol Toxicol 2:953–963
Mac Carthy DJ, Waud WR, Struck RF, Hill DL (1985) Disposition and metabolism of aniline in Fisher 344 rats and C57BL/6xC3H F1 mice. Cancer Res 45:174–180
Maier-Bode H, Hartel K (1981) Linuron and monolinuron. Residue Rev 77:1–364
Metcalfe CD (1988) Experimental induction of liver tumours in rainbow trout (Salmo gairdneri) by contaminated sediment from Hamilton harbour, Ontario. Can J Fish Aquat Sci 45:2161–2167
Metcalfe CD, Sonstegard RA (1984) Microinjection of carcinogens into rainbow trout embryos: An in vivo carcinogenesis assay. J Natl Cancer Inst 73:1125–1132
— — (1985) Oil refinery effluents: Evidence of cocarcinogenic activity in the trout embryo microinjection assay. J Natl Cancer Inst 75:1091–1097
Metcalfe CD, Cairns VW, Fitzsimmons JD (1988) Microinjection of rainbow at the sac-fry stage: A modified trout carcinogenesis assay. Aquat Toxicol 13:347–356
Nagel R, Bresch H, Caspers N, Hansen PD, Markert M, Munk R, Scholz N, Ter Höfte BB (1991) Effect of 3,4-dichloroaniline on the early life stages of the zebrafish (Brachydanio rerio): Results of a comparative laboratory study. Ecotox Environ Safety 21:157–164
Neskovic NK, Elezovic I, Karan V, Poleksic V, Budimir M (1993) Acute and subacute toxicity of atrazine to carp (Cyprinus carpio L.). Ecotox Environ Safety 25:173–182
Norrgren L, Andersson T, Björk M (1993) Liver morphology and cytochrome P450 activity in fry of rainbow trout after microinjection of lipid-soluble xenobiotics in the yolk-sac embryos. Aquat Toxicol 26:307–316
Oulmi Y, Negele RD, Braunbeck T (1995a) Cytopathology of liver and kidney in rainbow trout (Oncorhynchus mykiss) after long-term exposure to sublethal concentrations of linuron. Dis Aquat Org 21:35–52
— — — (1995b) Segment-specificity of the cytological response in rainbow trout (Oncorhynchus mykiss) renal tubules following prolonged exposure to sublethal concentrations of atrazine. Ecotox Environ Safety (in press)
Parodi S, Pala M, Russo P, Zunino A, Balbi C, Albini A, Valeri F, Cimberle MR, Santi L (1982a) DNA damage in liver, kidney, bone marrow, and spleen of rats and mice treated with commercial and purified aniline as determined by alkaline elution assay and sister chromatid exchange induction. Cancer Res 42:2277–2283
Parodi S, Taningher M, Boero P, Santi L (1982b) Quantitative correlations amongst alkaline DNA fragmentation, DNA covalent binding, mutagenicity in the Ames test, and carcinogenicity for 21 compounds. Mut Res 93:1–24
Rankin GO, Yang DJ, Cressy-Veneziano K, Casto S, Wang RT, Brown PI (1986) In vivo and in vitro nephrotoxicity of aniline and its monochlorophenyl derivatives in the Fischer-344 rat. Toxicology 38:269–283
Reimschuessel R, Bennett RO, May EB, Lipsky MM (1989) Renal histopathological changes in the goldfish (Carassius auratus) after sublethal exposure to hexachlorobutadiene. Aquat Toxicol 15:169–180
Reimschuessel R, Bennett RO, May EB, Lipsky MM (1990a) Development of newly formed nephrons in the goldfish kidney following hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol 18:32–38
— — — (1990b) Renal tubular cell regeneration, cell proliferation, and chronic nephrotoxicity in the goldfish Carassius auratus following exposure to a single sublethal dose of hexachlorobutadiene. Dis Aquat Org 8:211–224
Rippen G (1995) Handbuch Umweltchemikalien. Ecomed Verlagsgesellschaft, Landsberg
Rojik I, Nemcsok J, Boross L (1983) Morphological and biochemical studies on liver, kidney, and gill of fishes affected by pesticides. Acta Biol Hung 34:81–92
Seim WK, Curtis LR, Glenn SW, Chapman GA (1984) Growth and survival of developing steelhead trout (Salmo gairdneri) continuously or intermittently exposed to copper. Can J Fish Awaut Sci 41:433–438
Suzuki T, Casida JE (1981) Metabolites of diuron, linuron, and methazole formed by liver microsomal enzymes and spinach plants. J Agric Food Chem 29:1027–1033
Walker MK, Hufnagle LCJR, Clayton MK, Peterson RE (1992) An egg injection method for assessing early life stage mortality of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 22:15–37
Weinberg ES (1992) Efficient microinjection of zebrafish eggs. Zebrafish Science Monitor: 4–5
Yamagani K (1988) Mechanisms of hatching in fish. In: Hoar WS, Randall DJ (eds) Fish physiology XI. Academic Press, NY, pp 447–499
Zahn T, Braunbeck T (1993) Isolated fish hepatocytes as a tool in aquatic toxicology: Sublethal effects of dinitro-o-cresol and 2,4-dinitrophenol. Sci Total Environ Suppl: 721–734
Zahn T, Hauck C, Braunbeck T (1993) Cytological alterations in fish fibrocytic R1 cells as an alternative test system for the detection of sublethal effects of environmental pollutants: A case-study with 4-chloroaniline. In: Braunbeck T, Hanke W, Segner H (eds) Fish in ecotoxicology and ecophysiology. VCH, Weinheim, pp 103–126
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oulmi, Y., Braunbeck, T. Toxicity of 4-chloroaniline in early life-stages of zebrafish (Brachydanio rerio): I. Cytopathology of liver and kidney after microinjection. Arch. Environ. Contam. Toxicol. 30, 390–402 (1996). https://doi.org/10.1007/BF00212299
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00212299