Abstract
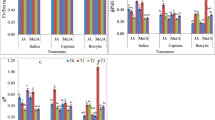
Jasmonic acid (JA) and its methyl ester (JA-Me) promoted the abscission of bean petiole expiants in the dark and light, and the activity of these compounds was almost same. JA and JA-Me did not enhance ethylene production in bean petiole expiants in the light, indicating that the abscission-promoting effects of these compounds are not the result of ethylene. Cells in the petiole adjacent to the abscission zone expanded during abscission but not in the pulvinus, and JA-Me promoted cell expansion in the petiole and the pulvinus. JA-Me had no effect on the total amounts of pectic and hemicellulosic polysaccharides in 2-mm segments of the abscission region, which included 1 mm of pulvinus and 1 mm of petiole from the abscission zone. On the other hand, the total amounts of cellulosic polysaccharides in this region were reduced significantly by the addition of JA-Me in the light. JA-Me had no effect on the neutral sugar composition of hemicellulosic polysaccharides during abscission. The decrease in the endogenous levels of UDP-sugars in the petiole adjacent to the abscission zone was accelerated during abscission by the addition of JA-Me in the light. Cellulase activities of pulvinus and petiole in 10-day-old seedlings were enhanced by the addition of JA. These results suggest that the promoting effect of JA or JA-Me on the abscission of bean petiole explants is due to the change of sugar metabolism in the abscission zone, in which the increase in cellulase activity involves the degradation of cell wall polysaccharides. Jasmonic acid (JA) and its methyl ester (JA-Me) are considered to be putative plant hormones for a number of reasons, including their wide occurrence in the plant kingdom, biologic, activities in multiple aspects at low concentrations, and their interaction with other plant hormones (for reviews see Parthier 1991, Hamberg and Gardner 1992, Sembdner and Parthier 1993, Ueda et al. 1994a). We have already reported that JA and JA-Me and C18-unsaturated fatty acids, which are considered to be the substrates of the biosynthesis of jasmonates, are powerful senescence-promoting substances (Ueda et al. 1982b, 1991a). Senescence symptoms induced by these compounds are identical to those of natural senescence. Recently we have also found that JA inhibited indole-3-acetic acid (IAA)-induced elongation of oat (Avena sativa L. cv. Victory) coleoptile segments by inhibiting the synthesis of cell wall polysaccharides (Ueda et al. 1994b, 1995). These facts led us to study the mode of actions of JA and JA-Me on promoting abscission, which is considered the last dramatic phenomenon of senescence. In this paper we report that JA and JA-Me promote abscission in bean (Phaseolus vulgaris L. cv. Masterpiece) petiole expiants and that the changes in the metabolism of cell wall polysaccharides in the petiole and the pulvinus adjacent to the abscission zone are involved in the promotive effects of these compounds.
Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- ACC:
-
1-aminocyclopropane-1-carboxylic acid
- DCB:
-
2,6-dichlorobenzonitrile
- HPLC:
-
high performance liquid chromatography
- IAA:
-
indole-3-acetic acid
- JA:
-
jasmonic acid
- JA-Me:
-
methyl jasmonate
- MES:
-
2-(N-morpholino)ethane-sulfonic acid, monohydrate
- TCA:
-
trichloroacetic acid
- Tris:
-
2-amino-2-hydroxymethy-1,3-propanediole
References
Albersheim P, Nevins D, English P, Karr A (1967) A method for the analysis of sugars in plant cell wall polysaccharides by gas-liquid chromatography. Carbohydr Res 5:340–345
Campillo E, Reid PD, Sexton R, Lewis LN (1990) Occurrence and localization of 9.5 cellulase in abscising and nonabscising tissues. Plant Cell 2:245–254
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Feingold DS, Avigad G (1980) Sugar nucleotide transformations in plants. In: Preiss J (ed) The biochemistry of plants. Vol 3. Academic Press, New York, pp 101–170
Hamberg M, Gardner HW (1992) Oxylipin pathway to jasmonates: Biochemistry and biological significance. Biochim Biophys Acta 1165:1–18
Hogetsu T, Shibaoka H, Shimokoriyama M (1974) Involvement of cellulose synthesis in action of gibberellin and kinetin on cellulose-synthesis inhibitor. Plant Cell Physiol 15:389–393
Inouhe M, Yamamoto R, Masuda Y (1987a) UDP-glucose level as a limiting factor for IAA-induced cell elongation in Avena coleoptile segments. Physiol Plant 69:49–54
Inouhe M, Yamamoto R, Masuda Y (1987b) Effects of indoleacetic acid and galactose on the UTP level and UDP-glucose formation in Avena coleoptile and Vigna epicotyl segments. Physiol Plant 69:579–585
Lewis LN, Koehler DE (1979) Cellulase in kidney bean seedlings. Planta 146:1–5
Loescher W, Nevins DJ (1972) Auxin-induced changes in Avena coleoptile cell wall composition. Plant Physiol 50:556–563
Meyer A, Miersch O, Büttner C, Dathe W, Sembdner G (1984) Occurrence of plant growth regulator jasmonic acid in plants. J Plant Growth Regul 3:1–8
Montezinos D, Delmer DP (1980) Characterization of inhibitors of cellulose synthesis in cotton fibers. Planta 148:305–311
Nishitani K, Masuda Y (1981) Auxin-induced changes in the cell wall structure: Changes in the sugar composition, intrinsic viscosity, and molecular weight distribution of matrix polysaccharides of the epicotyl cell wall of Vigna angularis. Physiol Plant 52:482–494
Osborne DJ (1968) Hormonal mechanisms regulating senescence and abscission. In: Wightman, F (ed) Biochemistry and physiology of plant growth substances. Runge Press, Ottawa, pp 815–840
Osborne DJ (1973) Internal factors regulating abscission. In: Kozlowsky TT (ed) Shedding of plant parts. Academic Press, New York, pp 125–147
Parthier B (1991) Jasmonates, new regulators of plant growth and development: Many factors and few hypotheses on their actions. Bot Acta 104:446–454
Rasmussen GK (1974) Cellulase activity in separation zones of citrus fruit treated with abscisic acid under normal and hypobaric atmospheres. J Am Soc Hort Sci 99:229–231
Reddy ASN, Friedmann M, Poovaiah BW (1988) Auxin-induced changes in protein synthesis in the abscission zone of bean explants. Plant Cell Physiol 29:179–183
Sembdner G, Parthier B (1993) The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol 44:569–589
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195: 19–23
Ueda J, Kato J (1981) Promotive effect of methyl jasmonate on oat leaf senescence in the light. Z Pflanzenphysiol 103:357–359
Ueda J, Kato J (1982a) Inhibition of cytokinin-induced plant growth by jasmonic acid and its methyl ester. Physiol Plant 54:249–252
Ueda J, Kato J (1982b) Abscisic acid and C18-unsaturated fatty acids as senescence-promoting substances from oat plants. J Plant Growth Regul 1:195–203
Ueda J, Kato J, Yamane H, Takahashi N (1981) Inhibitory effect of methyl jasmonate and its related compounds on kinetin-induced retardation of oat leaf senescence. Physiol Plant 52:305–309
Ueda J, Miyamoto K, Aoki M (1994b) Inhibitory effect of jasmonic acid on IAA-induced elongation of oat coleoptile segments and its possible mechanism in the metabolism of cell wall polysaccharides. Plant Cell Physiol 35:1065–1070
Ueda J, Miyamoto K, Kamisaka S (1994a) Separation of a new type of plant growth regulator, jasmonates, by chromatographic procedures. J Chromatogr A 658:129–142
Ueda J, Miyamoto K, Kamisaka S (1995) Inhibition of the synthesis of cell wall polysaccharides in oat coleoptile segments by jasmonic acid: Relevance to its growth inhibition. J Plant Growth Regul 14:69–76
Ueda J, Miyamoto K, Sato T, Momotani Y (1991b) Identification of jasmonic acid from Euglena gracilis Z as a plant growth regulator. Agric Biol Chem, 55:275–276.
Ueda J, Monta Y, Kato J (1991a) Promotive effect of C18 unsaturated fatty acids on the abscission of bean petiole expiants. Plant Cell Physiol 32:983–987
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ueda, J., Miyamoto, K. & Hashimoto, M. Jasmonates promote abscission in bean petiole expiants: Its relationship to the metabolism of cell wall polysaccharides and cellulase activity. J Plant Growth Regul 15, 189–195 (1996). https://doi.org/10.1007/BF00190583
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00190583