Abstract
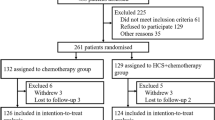
In order to examine the efficacy of adjuvant chemotherapy employing Mitomycin C (MMC) and carmofur (HCFU) for patients with noncuratively resected colorectal carcinoma, a cooperative study was performed by 54 institutions in the Kyushu and Chugoku areas in Japan. The prospective randomized controlled study consisted of two groups, one receiving only MMC and the other receiving MMC as well as HCFU. Out of an original total of 200, 170 cases were evaluable. Concerning the 30-month survival rate, a better result was observed in the MMC+HCFU group than in the MMC only group (Z-test: p<0.05). Significantly better survival rates were obtained in those cases with disseminating peritoneal metastasis, hepatic metastasis and Stage V cancer in the MMC+HCFU group as when compared with the MMC only group (generalized Wilcoxon test: p<0.05). No significant side effects due to the combined administration of HCFU were recognized. The combined administration of MMC and HCFU were recognized. The combined administration of MMC and HCFU was suggested to be a safe and effective adjuvant chemotherapy in noncuratively resected cases of colorectal carcinoma.
Similar content being viewed by others
References
Hoshi A, Iigo M, Nakamura A, Yoshida M, Kuretani K. Antitumor activity of 1-hexylcarbamoyl-5-fluorouracil in a variety of experimental tumors. Gann 1976; 67: 725–731.
Miura T, Uchiyama T, Takahashi M, Yamamoto K, Koizumi S, Fukamatsu K, Saito J. Physico-chemical properties and stabilities of 1-Hexylcarbamoyl-5-fluorouracil (HCFU). Iyakuhin Kenkyu 1980; 11: 73–81. (in Japanese)
Fujita H. Fluoropyrimidines (No 3) Carmofur. Pharma Medica 1985; 3: 112–123.
Koyama Y, Hattori T, Inokuchi K. (Cooperative Study Group of HCFU) 1-hexylcarbamoyl-5-fluorouracil (HCFU)-a masked 5-fluorinated pyrimidine. Cancer Treatment Reviews 1981; 8:147–156.
Japanese Research Society for cancer of Colon and Rectum: General rules for clinical and pathological studies on cancer of colon, rectum and anus. Jpn J Surg 1983; 13: 557–573.
Holden WD, Dixon WJ, Kuzma JW. The use of triethylenethiophosphoramide as an adjuvant to the surgical treatment of colorectal carcinoma. Ann Surg 1967; 165: 481–503.
Dixon WJ, Longmire WP, Holden WD. Use of triethylenethiophosphoramide as an adjuvant to the surgical treatment of gastric and colorectal carcinomas. Ann Surg 1971; 173: 26–39.
Higgins GA, Dwioht RW, Smoth JV. Fluorouracil as an adjuvant to surgery in carcinoma of the colon. Arch Surg 1971; 102: 339–343.
Imanaga H, Kato K. Adjuvant chemotherapy for colorectal cancer. In: Jinnai D, Murakami T, eds. Choushujutsu no Subete. Tokyo: Kanehara & Co. Ltd. 1978; 2: 657–666. (in Japanese)
Hattori T, Mori S, Hirata K, Ito I. Five year survival rate of gastric cancer patients treated by gastrectomy, large dose of mitomycin C, and/or allogenic bone marrow transplantation. Gan no Rinsho 1973; 19: 241–248. (in Japanese)
Tsuruo T, Iida M, Nakamura K, Tsukagoshi S, Sakurai Y. Inhibition of murine colon adenocarcinomas and Lewis Lung carcinoma by 1-hexylcarbamoyl-5-fluorouracil. Cancer Chemother Pharmacol 1980; 4: 83–87.
Koyama Y, Koyama Y. Phase I study of a new antitumor drug 1-hexycarbamoyl-5-fluorouracil (HCFU), administered orally: An HCFU clinical study group report. Cancer Treat Rep 1980; 64: 861–867.
Koyama Y, Inokuchi K, Koyama Y. (HCFU Clinical Study Group) Absorption and excretion of a new oral antitumor drug, 1-hexylcarbamoyl-5-fluorouracil (HCFU), in cancer patients. Jap J Clin Oncol 1980; 10: 83–92.
Fujita H, Ogawa K.In vivo distribution and activation of 1-hexylcarbamoyl-5-fluorouracil (HCFU). Rinsho Yakuri 1981; 12: 73–83. (in Japanese)
Shiraishi K, Kubo Y, Majima Y, Sato K, Sakami Y, Sakai T, Hirai K, Ninomiya F, Abe M, Tanikawa K. Clinical evaluation of 1-hexylcarbamoyl-5-fluorouracil (HCFU) in hepatocellular carcinoma. Gan to Kagakuryoho 1984; 11: 2348–2355. (English Abst.)
Watanabe A, Higashi T, Kobayashi M, Nakatsukasa H, Fujiwara M, Shioda T, Yamauchi Y, Ito T, Yamamoto H, Nagashima H. Role of oral administration of 1-hexylcarbomyl-5-fluorouracil (HCFU) for multimodel treatment of inoperable cirrhotics with hepatocellular carcinoma. Gan to Kagakuryoho 1984; 11: 2506–2512. (English Abst.)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Niimoto, M., Hattori, T., Tamada, R. et al. Mitomycin C plus carmofur (HCFU) adjuvant chemotherapy for noncuratively resected cases of colorectal carcinoma (interim report). The Japanese Journal of Surgery 17, 354–361 (1987). https://doi.org/10.1007/BF02470634
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02470634