Summary
The purine analogues O6-methylguanine and O6-benzylguanine are well-known as a chemical modulator of the DNA repair enzyme O6-methylguanine-DNA methyltransferase. Inactivation of the enzyme by O6-methylguanine or O6-benzylguanine is expected to enhance sensitivity of tumours to chloroethylnitrosoureas.
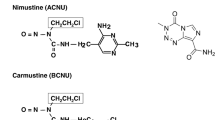
We studied the effect of O6-methylguanine or O6-benzylguanine pretreatment on cytotoxity of 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea hydrochloride (ACNU) in brain tumour cells and transplanted brain tumours. Two-hour exposure of O6-methylguanine at higher concentrations (500 μM, 1,000 μM) increased ACNU cytotoxicity by only 2 times in ACNU-resistant C6-1 brain tumour cells. O6-Benzylguanine at concentrations between 10 and 100 μM markedly enhanced the cytotoxic effecct. The ACNU sensitivity of the tumour cels pretreated with O6-benzylguanine was 5–40 times that of the cells without O6-benzylguanine. Neither O6-methylguanine nor O6-benzylguamne appreciably enhanced ACNU cytotoxicity of 9 L cells, which were origininally sensitive to ACNU. Intracarotid ACNU with O6-methylguanine or O6-benzylguanine decreased proliferating activity of transplanted C6-1 brain tumours significantly during 48 hours. O6-Benzylguanine pretreatment resulted in a greater degree of suppression for a long time. The C6-1 tumours treated only with intracarotid ACNU showed a transient inhibition and a rapid regrowth during 24 hours after the treatment.
These results indicate that O6-methylguanine or O6-benzylguanine increases ACNU cytotoxicity and may be feasible for effective combination therapy with chloroethylnitrosourea in the chemotherapy of malignant brain tumours.
Similar content being viewed by others
References
Abbott PJ, Saffhill R (1979) DNA synthesis with methylated poly (dC-dG) templates. Evidence for a competitive nature to miscoding by O6-methylguanme. Biochim Biophys Acta 562: 51–61
Balsiger RW, Montgomery JA (1960) Synthesis of potential anticancer agents. XXV. Preparation of 6-alkoxy-2-aminopurines. J Org Chem 25: 1573–1575
Benda P, Lightbody J, Sato G, Levine L, Sweet W (1968) Differentiated rat glial cell strain in tissue culture. Science 161: 370–371
Bodell WJ, Rupniak HTR, Rasmussen J, Morgan WF, Rosenblum ML (1984) Reduced level of DNA crosslinks and chromatid exchanges in 1,3-bis(2-chloroethyl)-1-nitrosourea resistant rat brain tumor cells. Cancer Res 44: 3763–3767
Bodell WJ, Aida T, Berger MS, Rosenblum ML (1986) Increased repair of O6-alkylguanine DNA adducts in glioma-derived human cells resistant to the cytotoxic and cytogenetic effects of 1,3-bis(2-chloroethyl)-1-nitrosourea. Carcinogenesis 7: 879–883
Day RS III, Ziolkowski CHJ, Scudiero DA, Meyer SA, Mattern MR (1980) Human tumor cell strains defective in the repair of alkylation damage. Carcinogenesis 1: 21–32
Deutsch M, Green SB, Strike TA, Burger PC, Robertson JT, Selker RG, Shapiro WR, Mealey J, Ransohoff J, Paoletti P, Smith KR, Odom GL, Hunt WE, Young B, Alexander E, Walker MD, Pistenmaa DA (1989) Results of a randomized trial comparing BCNU plus radiotherapy, streptozotocin plus radiotherapy, BCNU plus hyperfractioned radiotherapy in the postoperative treatment of malignant glioma. Int J Radiat Oncol Biol Phys 16: 1389–1396
Dexter EU, Yamashita TS, Donovan C, Gerson SL (1989) Modulation of O6-alkylguanine-DNA alkyltransferase in rats following intravenous administration of O6-methylguanine. Cancer Res 49: 3520–3524
Dolan ME, Morimoto K, Pegg AE (1985) Reduction of O6-alkylguanine-DNA alkyltransferase activity in HeLa cells treated with O6-alkylguanine. Cancer Res 45: 6413–6417
Dolan ME, Young GS, Pegg AE (1986) Effects of O6-alkylguanine pretreatment on the sensitivity of human color tumor cells to the cytotoxic effects of chloroethylating agents. Cancer Res 46: 4500–4504
Dolan ME, Pegg AE, Hora NK, Erickson LC (1988) Effect of O6-methylguanine on DNA interStrand cross-link formation by chloroethylnitrosourea and 2-chloroethyl(methylsulfonyl)methanesulfonate. Cancer Res 48: 3603–3606
Dolan ME, Moschel RC, Pegg AE (1990) Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci USA 87: 5368–5372
Dolan ME, Stine L, Mitchell RB, Moschel RC, Pegg AE (1990) Modulation of mammalian O6-alkylguanine-DNA alkyltransferase in vivo by O6-benzylguanine and its effect on the sensitivity of a human glioma tumor to 1-(2-chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea. Cancer Commun 2: 371–377
Erickson LC, Laurent G, Sharkey NA, Kohn KW (1980) DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature 288: 727–729
Frihart CR, Leonard NJ (1973) Allylic rearrangement from O6 to C-8 in the guanine series. J Am Chem Soc 95: 7174–7175
Fujio C, Chang HR, Tsujimura T, Ishizaki K, Kitamura H, Ikenaga M (1989) Hypersensitivity of human tumor xenografts lacking O6-alkylguanine-DNA alkyltransferase to the anti-tumor agent 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea. Carcinogenesis 10: 351–356
Gerson SL, Trey JE, Miller K (1988) Potentiation of nitrosourea cytotoxicity in human leukemic cells by inactivation O6-alkylguanine-DNA alkyltransferase. Cancer Res 48: 1521–1527
Hansen MB, Nielsen SE, Berg K (1989) Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119: 203–210
Hochberg FH, Pruitt AA, Beck DO, DeBrun G, Davis K (1985) The rationale and methodology fro intra-arterial chemotherapy with BCNU as treatment for glioblastoma. J Neurosurg 63: 876–880
Hori T, Muraoka K, Saito Y, Sasahara K, Inagaki H, Inoue Y, Adachi S, Anno Y (1987) Influence of models of ACNU administration on tissue and blood concentration in malignant brain tumors. J Neurosurg 66: 372–378
Hoshino T, Nagashima T, Murovic JA, Wilson CB, Edwards MSB, Gutin PH, Davis RL, DeArmond J (1986) In situ cell kinetics studies on human neuroectodermal tumors with bromodeoxyuridine labeling. J Neurosurg 64: 453–459
Ikenaga M, Tsujimura T, Chang HR, Fujio C, Zhang YP, Ishizaki K, Kataoka H, Shima A (1987) Comparative analysis of O6-methylguanine methyltransferase activity and cellular sensitivity to alkylating agents in cell strains derived from a variety of animal species. Mutat Res 184: 161–168
Kacinski BM, Rupp WD, Ludlum DB (1985) Repair of haloethylnitrosourea-induced DNA damage in mutant and adapted bacteria. Cancer Res 45: 6471–6474
Lindahl T, Sedgwick B, Sekiguchi M, Nakabeppu Y (1988) Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem 57: 133–157
Loew F, Papavero L (1988) The intraarterial route of drug delivery in the chemotherapy of malignant brain tumours. In: Symon Let al (eds) Advances and technical standards in neurosurgery, Vol. 16. Springer, Wien New York, pp 51–79
Mahaley MS (1991) Neuro-oncology index and review (adult primary brain tumors). J Neurooncol 11: 85–147
Mineura K, Fushimi S, Kowada M, Isowa G, Ishizaki K, Ikenaga M (1990) Linkage between O6-methylguanine-DNA methyltransferase (O6-MT) activity and cellular resistance to antitumor nitrosoureas in cultured rat brain tumour cell strains. Acta Neurochir (Wien) 103: 62–66
Mineura K, Watanabe K, Izumi I, Kowada M (1992) Modulation of BUdR labeling index in rat brain tumors following intracarotid ACNU administration. J Neurooncol 14: 201–205
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63
Pegg AE (1978) Enzymatic removal of O6-methylguanine from DNA by mammalian cell extracts. Biochem Biophys Res Commun 84: 166–173
Pegg AE (1984) Methylation of the O6 position of guanine in DNA is the most likely initiating event in carcinogenesis by methylating agents. Cancer Invest 2: 223–231
Pegg AE, Dolan ME (1987) Properties and assay of mammalian O6-alkylguanine-DNA alkyltransferase. Pharmacol Ther 34: 167–179
Pegg AE, (1990) Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenesis and therapeutic agents. Cancer Res 50: 6119–6129
Saffhill R, Margison GP, O'Connor PJ (1985) Mechanisms of carcinogensis induced by alkylating agents. Biochim Biophys Acta 823: 111–145
Sariban E, Kohn KW, Zlotogorski C, Laurent G, D'Incalci M, Day R III, Smith BH, Kornblith PL, Erickson LC (1987) DNA cross-linking responses of human malignant glioma cell strains to chloroethylnitrosoureas, cisplatin, and diaziquone. Cancer Res 47: 3988–3994
Schmidek HH, Nielsen SL, Schiller AL, Messer J (1971) Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg 34: 335–340
Scudiero DA, Mayer SA, Clatterbuck BE, Mattern MR, Ziolkowski CHJ (1984) Sensitivity of human cell strains having different abilities to repair O6-methylguanine in DNA to inactivation by alkylating agents including chloroethylnitrosoureas. Cancer Res 44: 2467–2474
Shapiro WR, Green SB, Burger PC, Mahaley MS, Selker RG, VanGilder JC, Robertson JT, Ransohoff J, Mealey J, Strike TA, Pistenmaa DA (1989) Randomized trial of three chemotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg 71: 1–9
Singer B (1984) Alkylation of the O6 of guanine is only one of many chemical events that may initiate carcinogenesis. Cancer Invest 2: 233–238
Takakura K, Abe H, Tanaka R, Kitamura K, Miwa T, Takeuchi K, Yamamoto S, Kageyama N, Handa H, Mogami H, Nishimoto A, Uozumi T, Matsutani M, Nomura K (1986) Effects of ACNU and radiotherapy on malignant glioma. J Neurosurg 64: 53–57
Tong WP, Kirk MC, Ludlum DB (1982) Formation of the crosslink 1-[N3-deoxycytidyl],2-[N1-deoxyguanosinyl]ethane in DNA treated with N,N′-bis(2-chloroethyl)-N-nitrosourea. Cancer Res 42: 3102–3105
Walker MD, Green SO, Bvar DP, Alexander E, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS, Owens G, Ransohoff J, Robertson JT, Shapiro WR, Smith KR, Wilson CB, Strike TA (1980) Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant gliomas after surgery. N Engl J Med 303: 1323–1329
Wiestler O, Kleihues P, Pegg AE (1984) O6-alkylguanine-DNA alkyltransferase activity in human brain and brain tumors. Carcinogenesis 5: 121–124
Yarosh DB (1985) The role of O6-methylguanine-DNA methyltransferase in cell survival, mutagenesis and carcinogenesis. Mutat Res 145: 1–16
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mineura, K., Izumi, I., Watanabe, K. et al. Potential of O6-methylguanine or O6-benzylguanine in the enhancement of chloroethylnitrosourea cytotoxicity on brain tumours. Acta neurochir 128, 13–20 (1994). https://doi.org/10.1007/BF01400647
Issue Date:
DOI: https://doi.org/10.1007/BF01400647