Summary
To investigate the influence of inducible nitric oxide synthase on cerebral arteries after subarachnoid haemorrhage (SAH) in vivo, lipopolysaccharide (LPS), a major inducer of inducible nitric oxide synthase, was injected intracisternally into control and SAH model dogs.
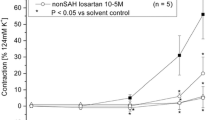
Intracisternal injection of LPS (0.5 mg) produced a long-lasting, submaximal vasodilation of the basilar artery of control dogs on angiography. This effect became significant at 4 hours after LPS injection and plateaued after 6 hours. This vasodilation was reduced by NG-monomethyl-L-arginine. Vasopressin slightly suppressed the vasodilation, while bradykinin increased it. The concentration of L-arginine in CSF decreased after LPS injection, while that of L-citrulline increased. In cytokines, the concentration of tumour necrosis factor-α (TNF-α) in CSF increased transiently at 4 hours after LPS injection, while interleukin-1β, interleukin-6, interferon-γ did not change. These data suggest that vasodilation by LPS is mainly due to nitric oxide predominantly synthesized by an inducible nitric oxide synthase, proximally induced by TNF-α.
Our data make it unlikely that SAH itself induces the inducible nitric oxide synthase in vascular tissue, since isolated endotheliumdenuded basilar artery from SAH model dogs did not respond to L-arginine. In SAH model dogs, the degree of vasodilation by LPS differed with the severity of vasospasm. Vasodilation was much greater in mild than in severe vasospasm in dogs, and was increased by superoxide dismutase. These findings suggest that the induction of inducible nitric oxide synthase or its activity may be less effective in severe vasospasm.
Similar content being viewed by others
References
Aisaka K, Gross SS, Griffith OW, Levi R (1989) NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun 160: 881–886
Bred DS, Hwang PM, Snyder SH (1990) Localization of nitric synthase indicating a neural role for nitric oxide. Nature 34 7: 768–770
Busse R, Mulsch A (1990) Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS 275: 87–90
Cowan CL, Cohen RA (1991) Two mechanisms mediate relaxation by bradykinin of pig coronary artery. NO-dependent and-independent responses. Am J Physiol 261: H830-H835
Emori T, Hirata Y, Kanno K, Marumo F (1993) Bradykinin stimulates endothelial nitric oxide (NO) production by calcium/calmodulin-dependent NO synthase. Hypertens Res 16: 131–137
Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL (1992) Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 257: 387–389
Fleming I, Julou-Schaeffer G, Gray GA, Parratt JR, Stoclet JC (1991) Evidence that an L-arginine/nitric oxide dependent elevation of tissue cyclic GMP content is involved in depression of vascular reactivity by endotoxin. Br J Pharmacol 103: 1047–1052
Fong Y, Tracey KJ, Moldawer LL, Hesse DG, Manogue KB, Kenney JS, Lee AT, Kuo GC, Allison AC, Lowry SF, Cerami A (1989) Antibodies to cachectin/TNF reduces interleukin −1β and interleukin-6 appearance during lethal bacteremia. J Exp Med 170: 1627–1633
Gardiner SM, Compton AM, Bennett T, Palmer RMJ, Moncada S (1990) Control of regional blood flow by endothelium-derived nitric oxide. Hypertension 15: 486–492
Gonzalez C, Fernandez A, Martin C, Moncada S, Estrada C (1992) Nitric oxide from endothelium and smooth muscle modulates responses to sympathetic nerve stimulation: implications for endotoxin shock. Biochem Biophys Res Commun 186: 150–156
Joly GA, Schini VB, Vanhoutte PM (1992) Balloon injury and interleukin-1β induce nitric oxide synthase activity in rat carotid arteries. Circ Res 71: 331–338
Kajita Y, Suzuki Y, Oyama H, Tanazawa T, Takayasu M, Shibuya M, Sugita K (1994) Combined effect of L-arginine and superoxide dismutase on the spastic basilar artery after subarachnoid hemorrhage in dogs. J Neurosurg 80: 476–483
Kanno K, Hirata Y, Imai T, Marumo F (1993) Induction of nitric oxide synthase gene by interleukin in vascular smooth muscle cells. Hypertension 22: 34–39
Koide M, Kawahara Y, Tsuda T, Yokoyama M (1993) Cytokine-induced expression of an inducible type of nitric oxide synthase gene in cultured vascular smooth muscle cells. FEBS 318: 213–217
Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, Dietzschold B (1993) In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA 90: 3024–3027
Lyons CR, Orloff GJ, Cunningham JM (1992) Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem 267: 6370–6374
Mathiesen T, Andersson B, Loftenius A, Holst HV (1993) Increased interleukin-6 levels in cerebrospinal fluid following subarachnoid hemorrhage. J Neurosurg 78: 562–567
Mollace V, Salvemini D, Anggard E, Vane J (1991) Nitric oxide from vascular smooth muscle cells: regulation of platelet reactivity and smooth muscle cell guanylate cyclase. Br J Pharmacol 104: 633–638
Moncada S, Palmer RMJ, Higgs EA (1989) Biosynthesis of nitric oxide from L-arginine: a pathway for the regulation of cell function and communication. Biochem Pharmacol 38: 1709–1715
Moritoki H, Takeuchi S, Hisayama T, Kondoh W (1992) Nitric oxide synthase responsible for L-arginine-induced relaxation of rat aortic rings in vitro may be an inducible type. Br J Pharmacol 107: 361–366
Nakaki T, Otsuka Y, Nakayama M, Kato R (1992) Endothelium-accelerated hyporesponsiveness of norepinephrine-elicited contraction of rat aorta in the presence of bacterial lipopolysaccharide. Eur J Pharmacol 219: 311–318
Nozaki K, Moskowitz MA, Maynard KI, Koketsu N, Dawson TM, Bredt DS, Snyder SH (1993) Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibers in cerebral arteries. J Cereb Blood Flow Metab 13: 70–79
Nunokawa Y, Ishida N, Tanaka S (1993) Cloning of inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem Biophys Res Commun 191: 89–94
Ochoa JB, Curti B, Peitzman AB, Simmons RL, Billiar TR, Hoffman R, Rault R, Longo DL, Urba WJ, Ochoa AC (1992) Increased circulation nitrogen oxide after human tumor immunotherapy: correlation with toxic hemodynamic change. J Natl Cancer Inst 84: 864–867
Oyama H, Suzuki Y, Satoh S, Hajita Y, Takayasu M, Shibuya M, Sugita K (1993) Role of nitric oxide in the cerebral vasodilatory responses to vasopressing and oxytocin in dogs. J Cereb Blood Flow Metab 13: 285–290
Palmer RMJ, Moncada S (1989) A novel citrulline-forming enzyme implicated in the formation of nitric oxide by endothelial cells. Biochem Biophys Res Commun 158: 348–352
Palmer RM, Bridge L, Foxwell NA, Moncada S (1992) The role of nitric oxide in endothelial cell damage and its inhibition by glucocorticoids. Br J Pharmacol 105: 11–12
Pittner RA, Spitzer JA (1992) Endotoxin and TNF-α directly stimulate nitric oxide formation in cultured rat hepatocytes from chronically endotoxemic rats. Biochem Biophys Res Commun 185: 430–435
Schaeffer GJ, Gray GA, Fleming I, Schott C, Parratt JR, Stoclet JC (1991) Activation of L-arginine-nitric oxide pathway is involved in vascular hyporeactivity induced by endotoxin. J Cardiovasc Pharmacol 17: 207–212
Schini VB, Junquero DC, Scott-Burden T, Vanhoutte PM (1991) Interleukin-1β induces the production of an L-argininederived relaxing factor from cultured smooth muscle cells from rat aorta. Biochem Biophys Res Commun 176: 114–121
Schott CA, Gray GA, Stoclet JC (1993) Dependence of endotoxin-induced vascular hyporeactivity on extracellular L-arginine. Br J Pharmacol 108: 38–43
Shapoval LN, Sagach VF, Pobegalio LS (1991) Nitric oxide ventrolateral medullary mechanisms of vasomotor control in the cat. Neurosci Lett 132: 47–50
Siren AL, Heldman E, Doron D, Lysko PG, Yue TL, Liu Y, Feuerstein G, Hallenbeck JM (1992) Release of proinflammatory and prothrombotic mediators in the brain and peripheral circulation in spontaniously hypertensive and normotensive Wistar-Kyoto rats. Stroke 23: 1643–1651
Smith REA, Palmer RMJ, Moncada S (1991) Coronary vasodilation induced by endotoxin in the rabbit isolated perfused heart is nitric oxide-dependent and inhibited by dexamethasone. Br J Pharmacol 104: 5–6
Suzuki Y, Satoh S, Kimura M, Oyama H, Asano T, Shibuya M, Sugita K (1992) Effects of vasopressin and oxytocin on canine cerebral circulation in vivo. J Neurosurg 77: 424–431
Suzuki Y, Satoh S, Oyama H, Takayasu M, Shibuya M (1993) Regional differences in the vasodilator response to vasopressin in canine cerebral arteries in vivo. Stroke 24: 1049–1054
Thiermermann C, Vane J (1990) Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol 182: 591–595
Thimermann C, Szabo C, Mitchell JA, Vane JR (1993) Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompression in hemorrhagic shock is mediated by nitric oxide. Proc Natl Acad Sci USA 90: 267–271
Toda N, Okamura T (1992) Different susceptibility of vasomotor nerve, endothelium and smooth muscle function to Ca++ antagonists in cerebral arteries. J Pharmacol Exp Ther 261: 234–239
Togashi H, Sakuma I, Yoshioka M, Kobayashi T, Yasuda H, Kitabatake A, Saito H, Gross SS, Levi R (1992) A central nervous system action of nitric oxide in blood pressure regulation. J Pharmacol Experi Therapeut 262: 343–347
Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A (1987) Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteriaemia. Nature 330: 662–664
Ueno M, Lee TJF (1993) Endotoxin decreases the contractile response of the porcine basilar artery to vasoactive substances. J Cereb Blood Flow Metab 13: 712–719
Vallance P, Collier J, Moncada S (1989) Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 28: 997–1000
Warren B, Coughlan ML, Williams TJ (1992) Endotoxininduced vasodilation in anaesthetized rat skin involves nitric oxide and prostaglandin synthesis. Br J Pharmacol 106: 953–957
Wiemer G, Scholkens BA, Becker RHA, Busse R (1991) Ramiprilat enhances endothelial autacoid formation by inhibiting breakdown of endothelium-derived bradykinin. Hypertension 18: 558–563
Yoshizumi M, Perrella MA, Burnett JC Jr, Lee ME (1993) Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res 73: 205–209
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tanazawa, T., Suzuki, Y., Anzai, M. et al. Vasodilation by intrathecal lipopolysaccharide of the cerebral arteries after subarachnoid haemorrhage in dogs. Acta neurochir 138, 330–337 (1996). https://doi.org/10.1007/BF01411745
Issue Date:
DOI: https://doi.org/10.1007/BF01411745