Abstract
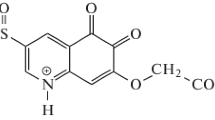
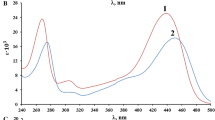
Mixed acidic constants (pK′ a ) of quinolinium oximes [1-(2-phenyl-2-hydroxyiminoethyl)-1-quinolinium chloride (F-1), 1-(2-phenyl-2-hydroxyiminoethyl)-1-isoquinolinium chloride (F-2), 1-(2-phenyl-2-hydroxyiminoethyl)-1-(4′-methyl)-quinolinium chloride (F-3), and 1-(2-phenyl-2-hydroxyiminoethyl)-1-(6′-methyl)-quinolinium chloride (F-4)] have been determined via their UV absorption spectra recorded in the series ofBritton-Robinson's buffer solutions in thepH region 8.74–11.28 (t=25±0.5°C, μ=0.2). The obtainedpK′ a values are in good agreement with those achieved by applying graphical methods. The followingpK′ a values have been obtained: 9.93 forF-1, 9.90 forF-2, and 10.02 forF-3 andF-4.
On the basis of potentiometric titrations thermodynamic acidic constants (pK a ) of compoundsF-1,F-2,F-3, andF-4 have been determined and they were found to be 9.82, 9.71, 9.91, and 9.86, respectively. The values obtained by transferringpK a intopK′ a are in good agreement with the values obtained spectrophotometrically.
Zusammenfassung
Die Mischaciditätskonstanten (pK′ a ) der Chinolin-Oxime 1-(2-Phenyl-2-hydroxyiminoethyl)-1-chinolinium chlorid (F-1), 1-(2-Phenyl-2-hydroxyiminoethyl)-1-isochinolium chlorid (F-2), 1-(2-Phenyl-2-hydroxyiminoethyl)-1-(4′-methyl)-chinolinium chlorid (F-3) und 1-(2-Phenyl-2-hydroxyiminoethyl)-1-(6′-methyl)-chinolinium chlorid (F-4) wurden durch ihre UV-Absorptionspektren in einer Reihe vonBritton-Robinson-Pufferlösungen impH-Intervall 8.74–11.28 (t=25±0.5°C; μ=0.2) bestimmt. Die berechnetenpK a -Werte stimmen mit den über graphische Methoden erhaltenen Ergebnissen überein. DerpK′ a -Wert beträgt 9.93 für die VerbindungF-1 und 9.90 fürF-2, sowie 10.02 fürF-3 andF-4.
Auf Grund der potentiometrischen Titration wurden auch die thermodynamischen Aciditätskonstanten (pK a ) berechnet: 9.82 fürF-1, 9.71 fürF-2, 9.91 fürF-3 und 9.86 fürF-4. Wenn man diese Konstanten in Mischaciditätskonstanten überträgt, erhält man Werte, die mit den durch spektrophotometrischen Bestimmungen erhaltenen Werten gut übereinstimmen.
Similar content being viewed by others
References
Binenfeld Z., Vojvodić V., Försvars medicin10, 114 (1974).
Engelhard N., Erdmann W. D., Arzneim. Forsch.14, 870 (1964).
Ashani J., Cohen S., J. Med. Chem.13, 471 (1970).
Grifantini M., Martelli S., Stein M. L., J. Pharm. Sci.58, 460 (1969).
Maksimović M., Arh. hig. rada toksikol.30, 227 (1979).
Malatesta P., Quaglia G., Floresta E., Farmaco (Ed. Sci.)23, 757 (1968).
Binenfeld Z., Bošković B., Rakin D., Ćosić M., Acta Pharm. Jugosl.21, 113 (1971).
Granov A., Maksimović M., Binenfeld Z., Nauchno-Tech. Pregled Nr. 2,34, 34 (1984).
Coch-Frugoni J. A., Gazz. Chim. Ital.87, 403 (1957).
Albert A., Serjeant E. P., The Determination of Ionization Constants, A Laboratory Manual. London: Chapman and Hall. 1971.
Stark J., Dissertation, Universität Freiburg, 1971.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karljiković, K.D., Stanković, B.S., Milosavljević, E.B. et al. Determination of acidic constants of some phenyl-hydroxyiminoethyl quinolinium compounds. Monatsh Chem 117, 661–670 (1986). https://doi.org/10.1007/BF00817903
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00817903