Summary
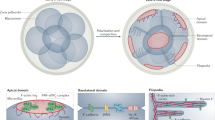
The distribution and arrangement of cytoskeletal components in the early embryo ofDrosophila melanogaster were examined by thin-section electron microscopy to elucidate their involvement in the formation of the cellular blastoderm, a process called cellularization. During the final nuclear division in the cortex of the syncytial blastoderm bundles of astral microtubules were closely associated with the surface plasma membrane along the midline where a new gutter was initiated. Thus the new gutter together with the pre-formed ones compartmentalized the embryo surface to reflect underlying individual daughter nuclei. Subsequently such gutters became deeper by further invagination of the plasma membrane between adjacent nuclei to form so-called cleavage furrows. Nuclei simultaneously elongated in the direction perpendicular to the embryo surface and numerous microtubules from the centrosomes ran longitudinally between the nucleus and the cleavage furrow. Microtubules often appeared to be in close association with the nuclear envelope and the cleavage furrow membrane. The plasma membrane at the advancing tip of the furrow was always undercoated with an electron-dense layer, which could be shown to be mainly composed of 5–6 nm microfilaments. These microfilaments were decorated with H-meromyosin to be identified as actin filaments. As cleavage proceeded, each nucleus with its perikaryon became demarcated by the furrow membrane, which then extended laterally to constrict the cytoplasmic connection between each newly forming cell and the central yolk region. The cytoplasmic strand thus formed possessed a prominent circular bundle of microfilaments which were also decorated with H-meromyosin and bidirectionally arranged, similar in structure to the contractile ring in cytokinesis. These observations strongly suggest that both microtubules and actin filaments play a crucial role in cellularization ofDrosophila embryos.
Similar content being viewed by others
References
Callaini G, Anselmi G (1988) Centrosome splitting during nuclear elongation in theDrosophila embryo. Exp Cell Res 178: 415–425
Campos-Ortega JA, Hartenstein V (1985) The embryonic development ofDrosophila melanogaster. Springer, Berlin Heidelberg New York Tokyo
Dan K (1988) Mechanism of equal cleavage of sea urchin egg: Transposition from astral mechanism to constricting mechanism. Zool Sci 5: 507–517
Ede DA, Counce SJ (1956) A cinematographic study of the embryology ofDrosophila melanogaster, Wilhelm Rouxs Arch Dev Biol 148: 402–415
Foe VE, Alberts BM (1983) Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation inDrosophila melanogaster. J Cell Sci 61: 31–70
Fullilove SL, Jacobson AG (1971) Nuclear elongation and cytokinesis inDrosophila montana. Dev Biol 26: 560–577
Hyman AA, White JG (1987) Determination of cell division axes in the early embryogenesis ofCaenorhabditis elegans. J Cell Biol 105: 2123–2135
Ishikawa H (1988) Plasmalemmal undercoat: The cytoskeleton supporting the plasmalemma. Arch Histol Cytol 51: 127–145
—, Bischoff R, Holtzer H (1969) Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol 43: 312–328
Karr TL, Alberts BM (1986) Organization of the cytoskeleton in earlyDrosophila embryos. J Cell Biol. 102: 1494–1509
Katoh K, Ishikawa H (1988) Electron microscopic observations on the contractile ring during cellularization inDrosophila melanogaster embryos. In: Proceedings 4th Asia-Pacific Conference and Workshop on Electron Microscopy, Bangkok, Thailand, pp 541–542
- (1988) The involvement of cytoskeleton in cellularization inDrosophila melanogaster embryos. In: Proceedings 4th Int Congr Cell Biol, Montreal, Canada, 167 (Abstr)
Kielhalt DP, Feghali R (1985) Cytoplasmic myosin fromDrosophila. J Cell Biol 101: 162 a (Abstr)
Mahowald AP (1963 a) Ultrastructural differentiation during formation of the blastoderm in theDrosophila melanogaster embryo. Dev Biol 8: 186–204
— (1963 b) Electron microscopy of the formation of the cellular blastoderm inDrosophila melanogaster. Exp Cell Res 32: 457–463
Rappaport R (1965) Geometrical relation of cleavage stimulus in invertebrate egg. J Theor Biol 9: 51–66
— (1969) Aster-equitorial surface relations and furrow establishment. J Exp Zool 171: 59–68
Rickoll WL (1976) Cytoplasmic continuity between embryonic cells and the primitive yolk sac during early gastrulationDrosophila melanogaster. Dev Biol 49: 304–310
—, Counce SJ (1980) Morphogenesis in the embryo ofDrosophila melanogaster-germ band extension. Wilhelm Rouxs Arch Dev Biol 188: 163–177
Schroeder TE (1968) Cytokinesis: filaments in the cleavage furrow. Exp Cell Res 53: 272–276
— (1972) The contractile ring II. Determining its brief existence, volumetric changes, and vital role in cleavingArbacia eggs. J Cell Biol 53: 419–434
— (1981) The origin of cleavage forces in dividing eggs-a mechanism in two steps. Exp Cell Res 134: 231–240
—, Otto J (1988) Immunofluorescent analysis of actin and myosin in isolated contractile rings of seas urchin eggs. Zool Sci 5: 713–725
Stafstrom JP, Staehelin LA (1984 a) Are annulate lamellae in theDrosophila embryo the result of overproduction of nuclear pore components? J Cell Biol 98: 699–708
— (1984 b) Dynamics of the nuclear envelope and of nuclear pore complexes during mitosis in theDrosophila embryo. Eur J Cell Biol 34: 179–189
Turner F, Mahowald AP (1976) Scanning electron microscopy ofDrosophila embryogenesis. I. The structure of the egg envelopes and the formation of the cellular blastoderm. Dev Biol 50: 95–108
Usui N, Yoneda M (1982) Ultrastructural basis of the tension increase in sea-urchin egg prior to cytokinesis. Dev Growth Differ 24: 453–465
Warn RM, Magrath R (1982) Observations by a novel method of surface changes during the syncytial blastoderm stage of theDrosophila embryo. Dev Biol 89: 540–548
— (1983) F-actin distribution during the cellularization of theDrosophila embryo visualized with FL-phalloidin. Exp Cell Res 143: 103–114
—, Warn A (1986) Microtubule arrays present during the syncytial and cellular blastoderm stage of the earlyDrosophila embryo. Exp Cell Res 163: 201–210
— —, Webb S (1984) Distribution of F-actin during cleavage of theDrosophila syncytial blastoderm. J Cell Biol 98: 156–162
—, Bullard B, Magrath R (1980) Changes in the distribution of cortical myosin during cellularization of theDrosophila embryo. J Embryol Exp Morphol 57: 167–176
—, Flegg L, Warn A (1987) An investigation of microtubules organization and functions in livingDrosophila embryos by injection of a fluorescently labeled antibody against tyrosinatedα-tubulin. J Cell Biol 105: 1721–1730
Wieschaus E, Nüsselin-Volhard C (1986)Drosophila, a practical approach. IRL Press, Oxford Washington
Wolf N, Regan CL, Fuller MT (1988) Temporal and spatial pattern of differences on microtubule behaviour duringDrosophila embryogenesis revealed by distribution of a tubulin isoform. Development 102: 311–324
Young P, Pesacreta T, Rose D, Kiehalt DP (1987) Immunofluorescent localization of cytoplasmic myosin inDrosophila melanogaster during embryonic development. J Cell Biol 105: 172a (Abstr)
Yoneda M, Dan K (1972) Tension at the surface of the dividing seaurchin egg. J Exp Biol 57: 575–587
Yonemura S, Kinoshita S (1986) Actin filament organization in the sand dollar egg cortex. Dev Biol 115: 171–183
Zalokar M, Erk (1976) Division and migration of nuclei during early embryogenesis ofDrosophila melanogaster. J Microsc Biol Cell 25: 97–106
— — (1977) Phase-partition fixation and staining ofDrosophila. Stain Technol 52: 89–95
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Katoh, K., Ishikawa, H. The cytoskeletal involvement in cellularization of theDrosophila melanogaster embryo. Protoplasma 150, 83–95 (1989). https://doi.org/10.1007/BF01403663
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01403663