Summary
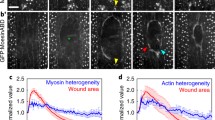
Growing hyphae of the oomyceteSaprolegnia ferax wounded by impalement with a ca. 0.2 μm diameter glass microelectrode normally respond within seconds with an apically directed cytoplasmic contraction followed by production of a plug which encases the electrode and occludes its recording of transmembrane potentials. This plug contains callose and Ca2+-associated membranes. To characterize the rapid wounding response, we disrupted specific filamentous (F) actin populations and Ca2+ regulation. Plug formation is inhibited by disruption of F-actin populations and low exogenous Ca2+ but not by inhibition of stretch-activated Ca2+ channels with Gd3+. Therefore, stretch-activated channels are not the immediate sensor. Instead, sensing may involve strain on the actin cytoskeleton which triggers the occlusion response. This wound response is qualitatively similar to the production of septa which isolate developing sporangia and seal severed hyphae, indicating the use of a normal basic cellular developmental system as a protective mechanism against environmental damage. The wound response is essential, since an inability to seal sites of mechanical damage is potentially catastrophic in acellular coenocytic organisms.
Similar content being viewed by others
Abbreviations
- APW:
-
artificial pond water
- BAPTA:
-
1,2-bis(orthzo-aminophenoxy)ethane-N,N,N′,N′-tetrapotassium acetate
- CTC:
-
chlortetracycline
- DIC:
-
Nomarski differential interference contrast microscopy
- F-actin:
-
filamentous actin
- LatB:
-
latrunculin B
- PM:
-
plasma membrane
- RP:
-
rhodamine-labeled phalloidin
- SA:
-
channels stretch-activated channels
References
Bachewich CL, Heath IB (1997) Differential cytoplasm-plasma membrane-cell wall adhesion patterns and their relationships to hyphal tip growth and organelle motility. Protoplasma 200: 71–86
— — (1998) Radial F-actin arrays precede new hypha formation in Saprolegnia: implications for establishing polar growth and regulating tip morphogenesis. J Cell Sci 111: 2005–2016
— — (1999) Cytoplasmic migrations and vacuolation are associated with growth recovery in hyphae ofSaprolegnia, and are dependent on the cytoskeleton. Mycol Res 103: 849–858
Bakker R, Dobbelmann J, Borst-Pauwels G (1986) Membrane potential in the yeastEndomyces magnusii measured by microelectrodes and TPP+ distribution. Biochim Biophys Acta 861: 205–209
Billon-Grand G, Marais M-F, Joseleau J-P, Girard V, Gay L, Fevre M (1997) A novel 1,3-β-glucan synthase from the oomyceteSaprolegnia monoica. Microbiology 143: 3175–3183
Caswell AH (1979) Methods of measuring intracellular calcium. Int Rev Cytol 56: 145–181
Currier HB (1957) Callose substance in plant cells. Am J Bot 44: 478–488
Foissner I (1988) The relationship of echinate inclusions and coated vesicles on wound healing inNitella flexilis (Characeae). Protoplasma 142: 164–175
— (1990) Wall appositions induced by ionophore A23187, CaCl2, LaCl3, and nifedipine in characean cells. Protoplasma 154: 80–90
—, Lichtscheidl I, Wasteneys G (1996) Actm-based vesicle dynamics and exocytosis during wound wall formation in characean internodal cells. Cell Motil Cytoskeleton 35: 35–48
Garrill A, Jackson SL, Lew RR, Heath IB (1993) Ion channel activity and tip growth: tip-localized stretch-activated channels generate an essential Ca2+ gradient in the oomyceteSaprolegnia ferax. Eur J Cell Biol 60: 358–365
Gay JL, Greenwood AD (1966) Structural aspects of zoospore production inSaprolegnia ferax with particular reference to the cell and vacuolar membranes. In: Madelin MF (ed) The fungus spore. Butterworths, London, pp 95–110 (Colston Papers, vol 18)
Goddard RH, La Claire JW II (1991) Calmodulin and wound healing in the coenocytic green algaErnodesmis verticuillata (Kützing) Børgensen: ultrastructure of the cortical cytoskeleton and immunogold labeling. Planta 186: 17–26
Gupta G, Heath IB (1997) Actin disruption by latrunculin B causes turgor-related changes in tip growth ofSaprolegnia hyphae. Fungal Genet Biol 21: 64–75
Heath IB (1975) Preservation of a labile cortical array of actin filaments in growing hyphal tips of the fungusSaprolegnia ferax. Eur J Cell Biol 44: 10–16
—, Greenwood AD (1970) The structure and formation of lomasomes. J Gen Microbiol 62: 129–137
—, Harold RL (1992) Actin has multiple roles in the formation and architecture of zoospores of the oomycetes,Saprolegnia ferax andAchlya bisexualis. J Cell Sci 102: 611–627
Hoch H (1977) Mycoparasitic relationships III: parasitism ofPhysalospora obtusa byCalcarisporium parasiticum. Can J Bot 55: 198–207
Hyde GJ, Heath IB (1997) Ca2+ gradients in hyphae and branches ofSaprolegnia ferax. Fungal Genet Biol 21: 238–251
Ince C, Ypey D, VanFurth R, Verveen A (1983) Estimation of the membrane potential of cultured macrophages from the fast potential transient upon microelectrode entry. J Cell Biol 96: 796–801
Jackson SL, Heath IB (1989) Effects of exogenous calcium ions on tip growth, intracellular Ca2+ concentration, and actin arrays in hyphae of the fungusSaprolegnia ferax. Exp Mycol 13: 1–12
— — (1990) Visualization of actin arrays in growing hyphae of the fungusSaprolegnia ferax. Protoplasma 154: 66–70
— — (1992) UV microirradiations elicit Ca2+ dependent apexdirected cytoplasmic contractions in hyphae. Protoplasma 170: 46–52
— — (1993) The dynamic behavior of cytoplasmic F-actin in growing hyphae. Protoplasma 173: 23–34
Kaminskyj SGW, Jackson SL, Heath IB (1992) Fixation induces differential polarized translocations of organelles in hyphae ofSaprolegnia ferax. J Microsc 167: 153–168
Kauss H (1987) Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol 38: 47–72
Kropf DL (1986) Electrophysiological properties ofAchlya hyphae: ionic currents studied by intracellular potential recording. J Cell Biol 102: 1209–1216
Levina NN, Lew RR, Heath IB (1994) Cytoskeletal regulation of ion channel distribution in the tip-growing organismSaprolegnia ferax. J Cell Sci 107: 127–134
Littlefleld L, Heath M (1979) Ultrastructure of rust fungi. Academic Press, New York
Manocha M, Golesorkhi R (1979) Host-parasite relations in a mycoparasite V: electron microscopy ofPiptocephalis virginiana infection in compatible and incompatible hosts. Mycologia 71: 565–576
—, Letourneau D (1978) Structure and composition of host-parasite interface in a mycoparasite system. Physiol Plant Pathol 12: 141–150
McKerracher LJ, Heath IB (1986) Polarized cytoplasmic movement and inhibition of saltations induced by calcium-mediated effects of microbeams in fungal hyphae. Cell Motil Cytoskelon 6: 136–145
Menzel D (1988) How do giant plant cells cope with injury? The wound response in siphonaceous green algae. Protoplasma 144: 73–91
Money NP, Harold FM (1992) Extension growth of the water moldAchlya: interplay of turgor and wall strength. Proc Natl Acad Sci USA 89: 4245–4249
— — (1993) Two water molds can grow without measurable turgor pressure. Planta 190: 426–430
Shackel KA, Polito VS, Ahmadi H (1991) Maintenance of turgor by rapid sealing of puncture wounds in leaf epidermal cells. Plant Physiol 97: 907–912
Swart HJ (1975) Callosities in fungi. Trans Br Mycol Soc 64: 511–515
Van Duijn B, Ypey DL, Van der Molen LG (1988) Electrophysiological properties ofDictyostelium derived from membrane potential measurements with microelectrodes. J Membr Biol 106: 123–134
Walker N (1955) Microelectrode experiments inNitella. Aust J Biol Sci 8: 476–489
Yuan S, Heath IB (1991a) A comparison of fluorescent membrane probes in hyphal tips ofSaprolegnia ferax. Exp Mycol 15: 103–115
— — (1991b) Chlortetracycline staining patterns of growing hyphal tips of the oomyceteSaprolegnia ferax. Exp Mycol 15: 91–102
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levina, N.N., Heath, I.B. & Lew, R.R. Rapid wound responses ofSaprolegnia ferax hyphae depend upon actin and Ca2+-involving deposition of callose plugs. Protoplasma 214, 199–209 (2000). https://doi.org/10.1007/BF01279064
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279064