Summary
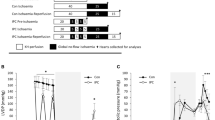
In a previous study we found that the development of fine structural alteration in atrial myocardium made ischaemicin vivo was slower than has been observed for ventricular myocardium. To explore possible reasons for this, parallel samples of atrial (A) and ventricular (V) myocardium undergoing autolysis (ischaemic necrosis)in vitro at 37°C were studied for up to 2 hours. At 15-minute intervals tissue was snap-frozen for measurement of pH, lactate, and adenine metabolites by HPLC. In half the experiments comparable specimens were taken for electron microscopic examination as well. Fine structural alteration developed less uniformly and more slowly in A than in V. The most striking metabolic differences between A and V were:
-
(1)
A had a consistently higher tissue pH and lower lactate level
-
(2)
The sum of the adenine + hypoxanthine metabolites was essentially constant bu significantly different for each (A=5.04±0.12 (s.e.m.), V=7.71±0.15 (s.e.m.) μmol/g wet tissue weight)
-
(3)
Initial ATP levels were lower (40% less) in A
-
(4)
The maximum accumulation of AMP was higher in A, despite its smaller pool of adenine metabolites
-
(5)
Both adenosine and inosine showed slower rates of change in A.
These results suggest that during early, severe ischaemic injury A and V show differing activities of 5′-nucleotidase.
Similar content being viewed by others
References
Armiger LC, Benson DC (1978) The fine structure of normal canine atrial myocardium with particular reference to mitochondria. J Mol Cell Cardiol 10:587–591
Armiger LC, Vanderwee MA, Fitzgerald S, Gavin JB, Herdson PB (1981) The effect of ischaemic injury on the fine structure of atrial myocardium. Pathology 13:345–355
Armiger LC, Seelye RN, Carnell VM, Smith CU, Gavin JB, Herdson PB (1976) Morphologic and biochemical changes in autolysing dog heart muscle. Lab Invest 34:357–362
Atkinson DE (1968) Energy charge of the adenylate pool as a regulatory parameter. Interaction of feedback modifiers. Biochemistry 7:4030–4034
De Wall RA, Vasko KA, Stanley EL, Kezdi P (1971) Responses of the ischaemic myocardium to allopurinol. Am Heart J 82:362–370
Ellis JP, Cain SM, Williams EW (1963) Rapid, accurate analysis of blood lactate. Technical documentary report SAM/TDR/63/49, U.S.A.F. School of Aerospace medicine. San Antonio, Texas
Fukuda Y (1975) Difference in calcium content of atrial and ventricular muscle. Jap J Physiol 25:467–479
Goldhaber SZ, Pohost GM, Kloner RA, Andrews E, Newell JB, Ingwall JS (1982) Inosine: A protective agent in an organ culture model of myocardial ischaemia. Circ Res 51:181–188
Hartwick RA, Assenza SP, Brown PR (1979) Identification and quantitation of nucleosides, bases and other UV-absorbing compounds in serum using reversed phase high-performance liquid chromatography. I Chromatographic Methodology. J Chromatog 186:647–658
Herdson PB, Kaltenbach JP, Jennings RB (1969) Fine structural and biochemical changes in dog myocardium during autolysis. Am J Pathol 57:539–557
Jaworek D, Gruber W, Bergmeyer HU (1974) Adenosine-5'-diphosphate and adenosine-5'-monophosphate. In: Methods of Enzymatic Analysis vol 4, Bergmeyer HU, ed. 2nd Eglis ed pp 2127–2131. Academic Press, New York
Jennings RB, Baum JH, Herdson PB (1965) Fine structural in myocardial ischaemic injury. Arch Pathol 79:135–143
Jennings RB, Hawkins HK, Lowe JE, Hill ML, Klotman S, Reimer KA (1978) Relation between high energy phosphate and letahl injury in myocardial ischaemia in the dog. Am J Pathol 92:187–214
Lamprecht W, Trautschold I (1974) Adenosine-5′-triphosphate. Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Methods of Enzymatic Analysis vol 4, Bergmeyer HU, ed, 2nd Englis ed pp 2101–2110. Academic Press, New York
Murat JC, Serfaty A (1974) Simple enzymatic determination of polysaccharide (glycogen) content of animal tissues. Clin Chem 20:1576–1577
Robinson DS (1952) Changes in the protein composition of chick muscle during development. Biochem J 52:521–628
Seelye RN, Carnell VM, Armiger LC (1976) A simple method for determining relative pH values and lactate levels in extracts of normal and ischemic or autolysing heart muscle. Biochem Med 16:187–194
Urthaler F, Walker AA, Hefner LL, James TN (1975) Comparison of contractile performance of canine atrial and ventricular muscles. Circ Res 37:762–771
Wikman-Coffelt J, Srivastava S (1979) Differences in atrial and ventricular myosin light chain LC1. FEBS Lett 106:207–212
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Armiger, L.C., Seelye, R.N., Morrison, M.A. et al. Comparative biochemistry and fine structure of atrial and ventricular myocardium during autolysis in vitro. Basic Res Cardiol 79, 218–229 (1984). https://doi.org/10.1007/BF01908308
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01908308