Abstract
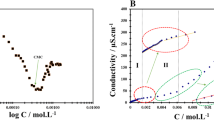
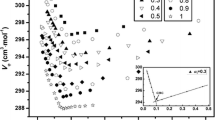
Two ternary phase diagrams of the cationic perfluorosurfactant diethanolheptadecafluoro-2-undecanolmethylammonium chloride (DEFUMAC) with an anionic perfluorosurfactant lithium perfluorooctanesulfonate (LiFOS) and an anionic hydrocarbon surfactant lithium dodecyl sulfate (LiDS) have been established at 25°C. The total surfactant concentration was less than 20wt%. In a wide mixing region of the LiFOS/DEFUMAC system, a lamellar-type phase,P β, was identified by its texture under a polarization microscope and by its x-ray diffraction pattern. Dispersed fragments ofP β-phase are present in the dilute solutions in which one surfactant was in excess. The anisotropy of electrical conductivity, flow birefringence, dynamic light scattering, and electric briefringence demonstrate that theP β fragments are disk-like with a radius of 0.7 μm. The disk-likeP β particles are transformed by shear into a spherical aggregate ofL α above a critical shear gradient. LiDS/DEFUMAC mixed solution forms dispersed and precipitatedL α in the dominant region. Radius and micropolarity of the dispersedL α aggregates are decreased as the ratio of LiDS:DEFUMAC approaches 1:1. On the basis of x-ray diffraction measurement the structure of precipitatedL α-phase seems to consist of monolayers.
Similar content being viewed by others
References
Chen DH, Hall DG (1973) Kolloid-Z u Z Polymere 251:41–44
Barker CA, Saul D, Tiddy GJT, Wheeler BA, Willis E (1974) J Chem Soc Faraday Trans II 70:154–162
Buckingham JH, Lucassen J, Hollway F (1978) J Colloid Interface Sci 67:423–431
Jokela P, Jönsson B, Wennerström H (1985) Progr Colloid Polym Sci 123:186–200
Malliaris A, Binana-Bimbele W, Zana R (1986) J Colloid Interface Sci 110:114–120
Jokela P, Jönsson B, Khan A (1987) J Phys Chem 91:3291–3298
Stellner KL, Amante JC, Scamehorn JF, Harwell JH (1988) J Colloid Interface Sci 123:186–200
Kato T, Iwai M, Seimiya T (1989) J Colloid Interface Sci 130:439–447
Kaler EW, Murthy AK, Rodriguez BE, Zasadzinski JAN (1989) Science 245:1371–1374
Fukuda H, Kawata K, Okuda H (1990) J Am Chem Soc 112:1635–1637
Winsor PA (1968) Chem Rev 68:1–40
Israelachivili JN, Mitchell DJ, Ninham BW (1976) J Chem Soc Faraday Trans II 72:1525–1568
Tiddy GJT (1980) Physics Reports (Review Section of Physics Letters) 57:1–46
Mitchell DJ, Ninham BW (1981) J Chem Soc Faraday Trans II 77:601–629
Jönsson B, Wennerström H (1981) J Colloid Interface Sci 80:482–496
Berr SS, Jones RRM (1989) J Phys Chem 93:2555–2558
Hoffmann H, Kalus J, Thurn H (1983) Colloid & Polymer Sci 261:1043–1049
Fontell K, Lindman B (1983) J Phys Chem 87:3289–3297
Hoffmann H (1984) Ber Bunsenges Phys Chem 88:1078–1093
Mukerjee P, Yang AYS (1976) J Chem Soc 80:1388–1390
Funasaki N, Hada S (1980) J Phys Chem 84:736–744
Shinoda K, Nomura T (1980) J Phys Chem 84:365–369
Meguro K, Ueno M, Suzuki T (1982) Yukagaku 31:909–914
Asakawa T, Johten K, Miyagishi S, Nishida M (1988) Langmuir 4:136–140
Tamori K, Esumi K, Meguro K (1991) J Colloid Interface Sci 142:236–243
Ootoshi S (1982) Reports Res Lab Asahi Glass Co Ltd 32:129–139
Rosevear FB (1954) JAOCS 31:628–639
Sato K, Mishima K (1984) Hyomen 22:579–593
Götz KG, Heckmann K (1958) J Colloid Sci 13:266–272
Rehage H, Wunderlich I, Hoffmann H (1986) Prog Colloid Polym Sci 72:51–59
Schorr W, Hoffmann H (1985) In: Corso XC (ed) Physics of Amphiphiles: Micelles, Vesicles and Microemulsions, p 160–180
Kalyanasundara K, Thomas JK (1977) J Phys Chem 81:2176–2180
Deguchi K, Mino J (1978) J Colloid Interface Sci 65:155–161
Janiak MJ, Small DM, Shiphey GG (1976) Biochemistry 15:1475–1480
Atkins PW (1990) In: Physical Chemistry 4th ed. Oxford University Press pp 961
Adam CD, Durrant JA, Lowry MR, Tiddy GJT (1984) J Chem Soc Faraday Trans I 80:789–801
Vincent JM, Skoulios AE (1966) Acta Cryst 20:432–440
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tamori, K., Kihara, K., Sanda, H. et al. Phase behavior and dynamic properties in mixed systems of anionic and cationic surfactants: lithium perfluorooctanesulfonate/diethanolheptadecafluoro-2-undecanolmethylammonium chloride (DEFUMAC) and lithium dodecyl sulfate/DEFUMAC aqueous mixtures. Colloid Polym Sci 270, 885–893 (1992). https://doi.org/10.1007/BF00657733
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00657733