Summary
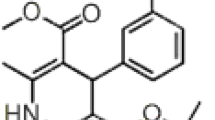
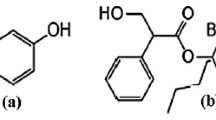
A rapid and specific reversed-phase high-performance liquid chromatographic method (RPHPLC) is described for the determination of mebeverine hydrochloride in tablets. Elution was performed on an octyl silane column with a methanol-water mixture (75-25), containing 0.05% hexylamine as silanol-blocking agent, adjusted to pH 5.0 with phosphoric acid. The method gave accurate, precise and reproducible results. The mean recovery of the drug from six synthetic tablet mixtures was 100.0% with a relative standard deviation (RSD) of 0.94%. In order to test the specificity of the method, the interference of the degradation compounds of mebeverine hydrochloride and of the intermediates from the synthesis was investigated. None of them did interfere. By means of mass spectrometry and UV-spectrophotometry, the degradation compounds of mebeverine were identified as veratric acid and as 4-|ethyl-[2-(4-methoxyphenyl)-1-methylethyl]amino| 1-butanol. The stability study proved that mebeverine hydrochloride is very stable in tablets; the tablets still contain more than 95% of the declared drug potency after storage for more than one year at 50°C.
Similar content being viewed by others
References
S. Czechowicz, M. W. McCulloch, C. Raper, M. J. Rand, Pharmacol. Res. Commun.1, 3 (1969).
F. C. Greenslade, C. K. Scott, K. L. Newquist, K. M. Krider, M. Chasin, J. Pharm. Sci.71, (1982).
G. Bertaccini, M. Impicciatore, E. Molina, L. Zappia, Farmaco, Ed. Sci.30, 10 (1975).
A. P. Launchbury, Progr. Med. Chem.7, (1970).
A. Kasahara, A. Akashi, T. Hashizume, K. Yamaguchi, Y. Shiroishi, Y. Oshima, Nippon Yakurigaku Zasshi64, 5 (1968).
M. Kanao, T. Hashizume, Y. Ichikawa, K. Irie, Y. Satoh, S. Isod, Chem. Pharm. Bull.,30, 1 (1982).
T. Daldrup, P. Michalke, W. Boehme, Chromatogr. Newsletter10, 1 (1982).
V. J. McLinden, A. M. Stenhouse, Forensic Sci. Int.13, 1 (1979).
4-[ N-[2-( p-Methoxyphenyl)-1-methylethyl)-N-ethylamino]-butyl methoxybenzoates synthesis. C. A.59, (1963).
A. Nahum, Cr. Horváth, J. Chromatogr.203, (1981).
K. E. Bij, Cs. Horváth, W. R. Melander, A. Nahum, J. Chromatogr.203, (1981).
B. A. Bidlingmeyer, J. K. Del Rios, J. Korpi, Anal. Chem.54, (1982).
Author information
Authors and Affiliations
Additional information
Colofac; Duspatal; Duspatalin
Rights and permissions
About this article
Cite this article
De Schutter, J.A., De Croo, F., Van der Weken, G. et al. Stability study and quantitative determination of mebeverine hydrochloride in tablets by means of reversed-phase high-performance liquid chromatography. Chromatographia 20, 185–192 (1985). https://doi.org/10.1007/BF02262709
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02262709