Summary
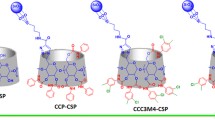
The enantiomers of primary amino compounds have previously been resolved on a chiral stationary phase (CSP) CSP-18C6I, prepared by immobilizing (+)-18-crown-6 tetracarboxylic acid. In this study related chiral stationary phases were prepared in an effort to broaden the scope of CSP18C6I. CSP-18C6II, synthesized to investigate the effect of spacer length, resolved the enantiomers of 2-amino-1,2-diphenylethanol and 1-(1-naphthyl)ethylamine (1-NEA) (hydrophobic amino compounds) with the largerk values and smaller α values than on CSP-18C6I, probably because of the greater hydrophobicity of CSP-18C6II. Use of CSP-18C6III, synthesized by modification of carboxylic acid functionality of CSP-18C6II by introduction of another chiral moiety,S-1-NEA, resulted in larger α values for 2-amino-1-phenylethanol and 2-amino-1-phenylpropanol than on CSP-18C6II, but the enantiomers of 1-NEA were not resolved, because of steric hindrance between 1-NEA and the chiral moiety. The amide derivativeN-3,5-dinitrobenzoyl-1-(α-naphthyl)ethylamine (DNN) as π-acceptor (3,5-dinitrobenzoyl function) or π-donor (naphthylethylamide function), and no primary amino functionality, was resolved on CSP-18C6III. The mechanism of separation of the enantiomers of DNN was assumed to be the π−π interaction between the 3,5-dinitrobenzoyl function (π-acceptor) of DNN and theS-1-NEA moiety (π-donor) of CSP-18C6III.
Similar content being viewed by others
References
L. R. Sousa, G. D. Y. Sogah, D. H. Hoffman, D. J. Cram, J. Am. Chem. Soc.100, 4569 (1978).
G. D. Y. Sogah, D. J. Cram, J. Am. Chem. Soc.101, 3035 (1979).
T. Shinbo, T. Yamaguchi, K. Nishimura, M. Sugiura, J. Chromatogr.405, 145 (1987).
T. Shinbo, T. Yamaguchi, H. Yanagishita, D. Kitamoto, M. Sugiura, J. Chromatogr.625, 101 (1992).
M. Hilton, D. W. Armstrong, J. Liq. Chromatogr.14, 9 (1991).
M. Okamoto, K. Takahashi, T. Doi, J. Chromatogr. A675, 2444 (1994).
Y. Walbroehl, J. Wagner, J. Chromatogr. A680,253 (1994).
R. A. Thompson, Z. Ge, N. Grinberg, D. Ellison, P. Tway, Anal. Chem.67, 1580 (1995).
Y. Makino, S. Ohta, M. Hirobe, Forensic Sci. Int.78, 65 (1996).
T. Pronce, B. Tilquin, J. Pharm. Biomed. Anal.14, 1175 (1996).
H. Nishi, K. Nakamura, H. Nakai, T. Sato, J. Chromatogr. A757, 225 (1997).
Y. Machida, H. Nishi, K. Nakamura, H. Nakai, T. Sato, J. Chromatogr. A805, 85 (1998).
Y. Machida, H. Nishi, K. Nakamura, J. Chromatogr. A830, 311 (1999).
Y. Machida, H. Nishi, K. Nakamura, J. Chromatogr. A810, 33 (1998).
Y. Machida, H. Nishi, K. Nakamura, Chirality11, 113 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Machida, Y., Nishi, H. & Nakamura, K. Separation of the enantiomers of amino and amide compounds on novel chiral stationary phases derived from a crown ether. Chromatographia 49, 621–627 (1999). https://doi.org/10.1007/BF02466903
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02466903