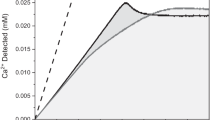
Abstract
THE problem of the physico-chemical constitution of the skeleton has received considerable attention in recent years1–3. Tracer experiments indicate that phosphorus-32 and calcium-45 appear promptly and in considerable proportions in the bony tissues, whence they are removed slowly4–6. These observations have led to an assumption that deposition of phosphorus-32 and calcium-45 in bony tissues is an exchange between bone and blood inorganic phosphate or calcium. Phosphorus-32 and calcium-45 have been used to study phosphate or calcium exchange between powdered bone and aqueous disodium hydrogen phosphate and calcium chloride solutions7–9. Different authors8,10,11 have concluded that the principal bone component is not tricalcium phosphate but rather hydroxylapatite to which phosphoric ions are adsorbed in such a quantity that the ratio of calcium to phosphorus is equal to 1 .94, which is the characteristic value for tricalcium phosphate. On the other hand, the findings of some workers1,2 support the idea of the existence of tricalcium phosphate in bony tissues. The experiments reported here are an attempt to study the exchange mechanism of both phosphate and calcium between pure tricalcium phosphate and radioactive phosphate or calcium solutions. These experiments were also performed in order to show to what extent the isotopic exchange method can help to elucidate a particular problem of structural chemistry. (Calcium-45 was prepared by neutron bombardment in the pile at the Clinton Laboratories, Oak Ridge, Terin., and phosphorus-32 at the pile at Harwell, Berks.)
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Brasseur, H., Dallemagne, M. J., and Melon, J., Nature, 157, 453 (1946).
Wallaeys, R., thesis, Paris (1952).
Posner, A. S., and Stephenson, S. R., Dental Res., 31, 371 (1952).
Hahn, L., Hevesy, G., and Lundsgaard, E. C., Biochem. J., 31, 1705 (1937).
Neuman, W. F., and Riley, R. F., J. Biol. Chem., 168, 545 (1947).
Govaerts, J., Dallemagne, M. J., and Melon, J., Endocrin., 48, 443 (1951).
Falkenheim, M., Neuman, W. F., and Hodge, H. C., J. Biol. Chem., 169, 713 (1947).
Neuman, W. F., and Murlyan, B. J., J. Biol. Chem., 185, 705 (1950).
Falkenheim, M., Underwood, E. E., and Hodge, H. C., J. Biol. Chem., 188, 805 (1951).
Hodge, H. C., Le Fevre, M. L., and Bale, W. F., Indust. Eng. Chem., 10, 156 (1938).
Manly, M. L., and Levy, S. R., J. Amer. Chem. Soc., 61, 2588 (1939).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GOVAERTS, J. Isotopic Exchanges and the Existence of Tricalcium Phosphate in Bone. Nature 174, 831–832 (1954). https://doi.org/10.1038/174831a0
Issue Date:
DOI: https://doi.org/10.1038/174831a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.