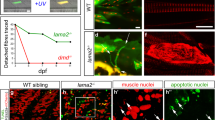
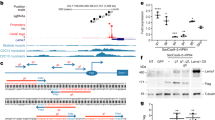
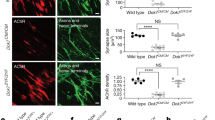
Abstract
Congenital muscular dystrophy is a heterogeneous and severe, progressive muscle-wasting disease that frequently leads to death in early childhood1,2. Most cases of congenital muscular dystrophy are caused by mutations in LAMA2, the gene encoding the α2 chain of the main laminin isoforms expressed by muscle fibres. Muscle fibre deterioration in this disease is thought to be caused by the failure to form the primary laminin scaffold, which is necessary for basement membrane structure3, and the missing interaction between muscle basement membrane and the dystrophin–glycoprotein complex (DGC)4 or the integrins5. With the aim to restore muscle function in a mouse model for this disease, we have designed a minigene of agrin, a protein known for its role in the formation of the neuromuscular junction6. Here we show that this mini-agrin—which binds to basement membrane7 and to α-dystroglycan8, a member of the DGC—amends muscle pathology by a mechanism that includes agrin-mediated stabilization of α-dystroglycan and the laminin α5 chain. Our data provides in vivo evidence that a non-homologous protein in combination with rational protein design can be used to devise therapeutic tools that may restore muscle function in human muscular dystrophies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tome, F. M. S., Guicheney, P. & Fardeau, M. in Neuromuscular Disorders: Clinical and Molecular Genetics (ed. Emery, A. E. H.) 21–57 (John Wiley & Sons, West Sussex, 1998).
Miyagoe-Suzuki, Y., Nakagawa, M. & Takeda, S. Merosin and congenital muscular dystrophy. Microsc. Res. Tech. 48, 181–191 (2000).
Colognato, H. & Yurchenco, P. D. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, 213–234 (2000).
Campbell, K. P. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell 80, 675–679 (1995).
Mayer, U. et al. Absence of integrin α7 causes a novel form of muscular dystrophy. Nature Genet. 17, 318–323 (1997).
Ruegg, M. A. & Bixby, J. L. Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci. 21, 22–27 (1998).
Denzer, A. J., Brandenberger, R., Gesemann, M., Chiquet, M. & Ruegg, M. A. Agrin binds to the nerve-muscle basal lamina via laminin. J. Cell Biol. 137, 671–683 (1997).
Gesemann, M., Brancaccio, A., Schumacher, B. & Ruegg, M. A. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J. Biol. Chem. 273, 600–605 (1998).
Patton, B. L., Miner, J. H., Chiu, A. Y. & Sanes, J. R. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol. 139, 1507–1521 (1997).
Ringelmann, B. et al. Expression of laminin α1, α2, α4, and α5 chains, fibronectin, and tenascin-C in skeletal muscle of dystrophic 129ReJ dy/dy mice. Exp. Cell Res. 246, 165–182 (1999).
Talts, J. F. et al. Structural and functional analysis of the recombinant G domain of the laminin α 4 chain and its proteolytic processing in tissues. J. Biol. Chem. 275, 35192–35199 (2000).
Denzer, A. J. et al. Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO J. 17, 335–343 (1998).
Burgess, R. W., Nguyen, Q. T., Son, Y. J., Lichtman, J. W. & Sanes, J. R. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron 23, 33–44 (1999).
Jaynes, J. B., Chamberlain, J. S., Buskin, J. N., Johnson, J. E. & Hauschka, S. D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol. Cell. Biol. 6, 2855–2864 (1986).
Sternberg, E. A. et al. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol. Cell. Biol. 8, 2896–2909 (1988).
Kuang, W. et al. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J. Clin. Invest. 102, 844–852 (1998).
Sunada, Y., Bernier, S. M., Kozak, C. A., Yamada, Y. & Campbell, K. P. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J. Biol. Chem. 269, 13729–13732 (1994).
Xu, H., Wu, X. R., Wewer, U. M. & Engvall, E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nature Genet. 8, 297–302 (1994).
Xu, H., Christmas, P., Wu, X. R., Wewer, U. M. & Engvall, E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc. Natl Acad. Sci. USA 91, 5572–5576 (1994).
Colognato, H. & Yurchenco, P. D. The laminin α2 expressed by dystrophic dy(2J) mice is defective in its ability to form polymers. Curr. Biol. 9, 1327–1330 (1999).
MacPike, A. D. & Meier, H. Comparison of dy and dy2J, two alleles expressing forms of muscular dystrophy in the mouse. Proc. Soc. Exp. Biol. Med. 151, 670–672 (1976).
Ibraghimov-Beskrovnaya, O. et al. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355, 696–702 (1992).
Ervasti, J. M., Kahl, S. D. & Campbell, K. P. Purification of dystrophin from skeletal muscle. J. Biol. Chem. 266, 9161–9165 (1991).
Henry, M. D. & Campbell, K. P. A role for dystroglycan in basement membrane assembly. Cell 95, 859–870 (1998).
Cote, P. D., Moukhles, H., Lindenbaum, M. & Carbonetto, S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nature Genet. 23, 338–342 (1999).
Meier, T. et al. A minigene of neural agrin encoding the laminin-binding and acetylcholine receptor-aggregating domains is sufficient to induce postsynaptic differentiation in muscle fibres. Eur. J. Neurosci. 10, 3141–3152 (1998).
Sorokin, L. M., Pausch, F., Durbeej, M. & Ekblom, P. Differential expression of five laminin alpha (1-5) chains in developing and adult mouse kidney. Dev. Dyn. 210, 446–462 (1997).
Schulze, B., Mann, K., Battistutta, R., Wiedemann, H. & Timpl, R. Structural properties of recombinant domain III-3 of perlecan containing a globular domain inserted into an epidermal-growth-factor-like motif. Eur. J. Biochem. 231, 551–556 (1995).
Herrmann, R. et al. Dissociation of the dystroglycan complex in caveolin-3-deficient limb girdle muscular dystrophy. Hum. Mol. Genet. 9, 2335–2340 (2000).
Turney, S. G., Culican, S. M. & Lichtman, J. W. A quantitative fluorescence-imaging technique for studying acetylcholine receptor turnover at neuromuscular junctions in living animals. J. Neurosci. Methods 64, 199–208 (1996).
Acknowledgements
We are grateful to M. Chiquet, S. Kröger, U. Mayer, L. Sorokin and R. Timpl for providing us with antibodies. We thank S. Arber, A. Brancaccio and T. Meier for discussions. M. Dürrenberger and U. Sauder for help in electron microscopy; J.-F. Spetz for DNA injection; M. Willem for providing us with the MCK promoter; and P. Scotton for the help with artwork. Special thanks go to D. Walz for his help in the statistical analysis of the results. This work was supported by grants from the Swiss National Science Foundation (MAR), the Kanton of Basel-Stadt (MAR), the Swiss Foundation for Research on Muscle Diseases (MAR and UM), from the National Institutes of Health (EE), the Novartis Research Foundation (UM), the Deutsche Forschungsgemeinschaft, Aktion Benni and company, and the Ernst und Berta Grimmke-Stiftung (HL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moll, J., Barzaghi, P., Lin, S. et al. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature 413, 302–307 (2001). https://doi.org/10.1038/35095054
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35095054
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.