Abstract
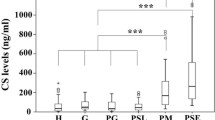
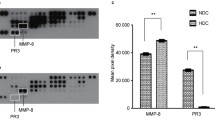
Cathepsin B (EC 3.4.22.1), a typical lysosomal cysteine proteinase was identified immunologically with anti-human cathepsin B antibody in inflammatory exudate, gingival crevicular fluid (GCF) of adult periodontitis patients. The sensitive enzyme immunoassay (EIA) system initially developed, was rarely influenced by the presence of endogenous, cysteine proteinase inhibitors, cystatin(s), indicating that it is possible to quantify the gross amount of cathepsin B including free enzyme forms and enzyme-inhibitor complex forms using this EIA system. The cathepsin B levels in, GCF as determined by EIA and the activity measured with Z-Arg-Arg-MCA showed positive and significant correlation with various clinical parameters. Immunoblotting analysis revealed that the molecular form was a 29 kDa mature enzyme. More than 95% of Z-Arg-Arg-MCA hydrolytic activity in each GCF sample was inhibited by CA-074, specific inhibitor of cathepsin B. These results strongly suggested that the gross amount of cathepsin B in GCF as well as its activity level is closely associated with the severity of the disease and that cathepsins B play an important role in the pathogenesis of periodontitis.
Similar content being viewed by others
References
Barrett AJ, Kirschke H, Cathepsin B, cathepsin H and cathepsin L. Meth Enzymol 1981;80:535–61.
Katunuma N, Kakegawa H, Matsunaga Y, Nikawa I, Kominami E. Different functional share of individual lysosomal cathepsins in normal and pathological conditions. In: Cheronis JC, Repine JE, editors. Proteases, protease inhibitors and protease-derived peptides. Basel: Birkhäuser, 1993:195–210.
Guinec N, Dalet-Fumeron V, Pagano M. In vitro study of basement membrane degradation by the cysteine proteinases, cathepsins B, B-like and L. Digestion of collagen IV, laminin, fibronectin and release of gelatinase activities from basement membrane fibronectin. Biol Chem Hoppe-Seyler 1993;374:1135–46.
Maciewicz RA, Wotton SF, Etherington DJ, Duannce VC, Susceptibility of the cartilage collagens types II, IX and XI to degradation by the cysteine proteinases, cathepsin B and L. FEBS Lett 1990;269:189–93.
Buck MR, Karustis DG, Day NA, Honn KV, Sloane BF. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tunour tissues. Biochem J 1992;282:273–8.
Eeckhout Y, Delaisse JM, Ledent P, Vaes G. The proteinases of bone resorption. In: Glauert AM, editor. The control of tissue damage. Amsterdam: Elsevier Science Publishers B.V. 1988;297–313.
Etherrington DJ, Maciewicz RA, Taylor MAJ, Wardale RJ. The role of collagen-degrading cysteine proteinases in connective tissue metabolism. In: Turk V, editor. Cysteine proteinases and their inhibitors. Berlin: Walter de Gruyter 1986;269–82.
Sloane BF, Lah TT, Day NA Rozhin J, Bando Y, Honn KV. Tumor cysteine proteinases and their inhibitors. In: Turk V, editor. Cysteine proteinases and their inhibitors. Berlin: Walter de Gruyter 1986;729–49.
Meheus LA, Fransen LM, Raymakers JG, Blockx HA, Van Beeumen JJ, Van Bun SM, et al. Identification by microsequencing of lipopolysaccharide-induced proteins secreted by mouse macrophages. J Immunol 1993;151:1535–47.
Katunuma N. Participation of lysosomal cathepsins in antigen processing and bone resorption. In: Katunuma N, Suzuki K, Travis J, Fritz J, editors. Biological functions of proteinases and inhibitors. Tokyo: Japan Scientific Societies Press 1994:3–21.
Jochum, M, Machleidt W, Fritz H. Proteolysis-induced pathomechanisms in acute inflammation and related therapeutic approaches. In: Cheronis JC, Repine JE, editors. Protease, protease inhibitors and Protease-Derived peptides. Agents Actions Suppl. Vol. 42. Basel: Birkhäuser 1993:51–69.
Meijers MHM, KoopdonK-Kool J, Meacock SCR, VanNoorden CJF, Bunning RAD, Billingham MEJ. Cysteine proteinase activity in the development of arthritis in an adjuvant model of the rat. Agents Actions 1993;39:C219–21.
Huet G, Flipo RM, Richet C, Thiebaut C, Demeyer D, Balduyck M, et al. Measurement of elastase and cysteine proteinases in synovial fluid of patients with, rheumatoid arthritis, sero-negative spondylarthropathies, and osteoarthritis. Clin Chem 1992;38:1694–7.
Kennett CN, Cox SW, Eley BM. Comparative histochemical, biochemical and immunocytochemical studies of cathepsin B in human gingiva. J Periodont Res 1994;29:203–13.
Eley BM, Cox SW. Cathepsin B- and L-Like activities at local gingival sites of chronic periodontitis patients. J Clin Periodontol 1991;18:499–504.
Eley BM, Cox SW. Cathepsin B/L, elastase-, tryptase-trypsin-and dipeptidyl peptidase IV-like activities in gingival crevicular fluid: correlation with clinical parameters in untreated chronic periodontitis patients. J. Periodont Res 1992;27:62–9.
Cox SW, Eley BM. Detection of cathepsin B- and L-, elastase-, tryptase-, trypsin-, and dipeptidyl peptidase-like activities in crevicular fluid from gingivitis and periodontitis patients with peptidyl derivatives of 7-amino-4 trifluoromethyl coumarin. J Periodont Res 1989;24:353–61.
Kunimatsu K, Yamamoto K, Ichimaru E, Kato Y, Kato I. Cathepsins B, H and L activities in gingival crevicular fluid from chronic adult periodontitis patients and experimental gingivitis subjects. J Periodont Res 1990;25:69–73.
Kadowaki T, Yoneda M, Okamoto K, Maeda K, Yamamoto K, Purification and characterization of a noval arginine-specific cysteine proteinase (argingipain) involved in the pathogenesis of periodontal disease from the culture supernatant of Porphyromonas gingivalis. J Biol Chem 1994;269:21371–8.
Bedi SG, Williams T. Purification and characterization of a collagen-degrading protease from Porphyromonas gingivalis. J Biol Chem 1994;269:599–606.
Chen Z, Potempa J, Polanowski A, Wikstrom M, Travis J. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. J Biol Chem 1992;267:18896–901.
Ichimaru E, Imura K, Hara Y, Kato Y, Kato I. Cystatin activity in gingival crevicular fluid from periodontal disease patents measured by a new quantitative analysis method. J Periodont Res 1992;27:119–125.
Hizawa K, Nonaka I, Sugita H, Kominami E, Katunuma N. Abnormal increase of lysosomal cysteine proteinases in rimmed vacuoles in the skeletal muscle. Am J Pathol 1986;122:193–8.
Ichimaru E, Sakai H, Saku T, Kunimatsu K, Kato Y, Kato I, et al. Characterization of hemoglobin-hydrolyzing acidic proteinases in human and rat neutrophils. J Biochem 1990;108:1009–15.
Udenfriend S, Stein S, Bohlen P, Dairman W. Fluorescamine: A reagent for assay of amino acid, peptides, proteins, and primary amines in the picomole range. Science 1972;178:871–2.
Barrett AJ. Cystatin, the egg white inhibitor of cysteine proteinases. Meth Enzymol 1981;80:711–78.
Barrett AJ, Rawling ND, Davies ME, Machleidt W, Salvesen G, Turk V. Cysteine proteinase inhibitors of the cystatin superfamily. In: Barrett AJ, Salvesen G, editors. Proteinase inhibitors. Amsterdam: Elsevier, 1986:515–69.
Towatari T, Nikawa T, Murata M, Yokoo C, Tamai M, Hanada K, et al. Novel epoxysuccinyl peptides. A selective inhibitor of cathepsin B, in vivo. FEBS Lett 1991;280:311–5.
Kominami E, Katunuma N. Biosynthesis processings and localizations of lysosomal cysteine proteinases. In: Katunuma N, Kominami E, editors. Intracellular Proteolysis. Tokyo: Japan Scientific Societies Press 1989:52–60.
Cimasoni G. Crevicular Fluid Updated. In: Myers HM, editor. Monographs in Oral Sciences. Basel: Karger 1983:1–152.
Author information
Authors and Affiliations
Additional information
accepted by W. B. van den Berg
Rights and permissions
About this article
Cite this article
Ichimaru, E., Tanoue, M., Tani, M. et al. Cathepsin B in gingival crevicular fluid of adult periodontitis patients: Identification by immunological and enzymological methods. Inflamm Res 45, 277–282 (1996). https://doi.org/10.1007/BF02280991
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02280991