Abstract
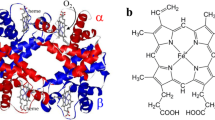
The homodimeric hemoglobin component present in the red cells of the bivalve molluscScapharca inaequivalvis, HbI, is endowed with high cooperativity in ligand binding. This behaviour is in contrast with that of vertebrate hemoglobins in which cooperativity is associated with a tetrameric assembly and the presence of two types of chain. Analysis of the aminoacid sequence and immunological data suggested that the assembly of HbI differed from that characteristic of vertebrate hemoglobins and hence that cooperativity had an unusual structural basis. Indeed the X-ray structures of the carbonmonoxy and deoxy derivatives at 2.4Å resolution showed that in HbI the heme carrying E and F helices are not exposed to solvent as in the vertebrate hemoglobin tetramer, but form the subunit interface and bring the two heme groups practically in direct contact through a network of hydrogen bonds. Ligand binding brings about marked structural changes that are limited to the heme environment, whereas quaternary changes are only minor. The structural changes in the heme environment result in alterations in the network of interactions between the heme groups which lead to changes in ligand affinity. In HbI therefore cooperativity in ligand binding is achieved through direct heme-heme communication as opposed to the long range information transfer operative in the vertebrate hemoglobin tetramer.
Similar content being viewed by others
References
Chiancone, E., Vecchini, P., Verzili D., Ascoli, F., and Antonini, E., J. molec. Biol.152 (1981) 577.
Petruzzelli, R., Goffredo B. M., Barra, D., Bossa F., Boffi A., Verzili, D., Ascoli F., and Chiancone E., FEBS Lett.184 (1985) 328.
Verzili, D., Citro, G., Ascoli, F., and Chiancone, E., FEBS Lett.181 (1985) 347.
Royer, W. E. Jr, Hendrickson, W. A., and Chiancone, E., J. biol. Chem.264 (1989) 21052.
Royer, W. E. Jr, Hendrickson, W. A., and Chiancone, E., Science249 (1990) 518.
Royer, W. E. Jr, J. molec. Biol.235 (1994) 657.
Chiancone, E., and Gibson, Q. H., J. biol. Chem.264 (1989) 21062.
Chiancone, E., Elber, R., Royer, W. E. Jr, Regan, R., and Gibson, Q. H., J. biol. Chem.268 (1993) 5711.
Rousseau, D. L., Song, S., Friedman, J. M. Boffi, A., and Chiancone, E., J. biol. Chem.268 (1993) 5719.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chiancone, E. Scapharca dimeric hemoglobin: A new mechanism of information transfer between globin chains. Experientia 51, 198–199 (1995). https://doi.org/10.1007/BF01931091
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01931091