Abstract
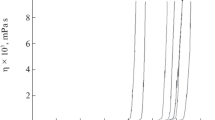
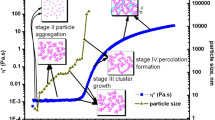
The formation and growth of polymeric particles during the hydrolysis and condensation of PbZr0.3Ti0.7 O3 (PZT 30/70) sol-gel precursor solutions have been investigated by using photon correlation spectroscopy (PCS), small angle X-ray scattering (SAXS) and by measuring their rheological properties. The measurements showed that the growth of the particles in the transition of PZT sol to gel followed a simple polymerisation process. Solution A (containing ‘by-products’) and Solution B (‘by-products’ removed) displayed a similar plot of logarithmic viscosity against logarithmic time, indicating that the particles in both solutions have similar structures after hydrolysis. The changes in viscosity and particle size with time were described by single logarithmic growth models. However, the increasing rate of logarithmic particle size in Solution B is higher than that in Solution A. A model for the form of the aggregates is discussed which is applicable to PZT organometal-particle aggregation process in systems with acetic acid as a modifier.
Similar content being viewed by others
References
J.F. Scott and C.A. Paz de Araujo, Science: Ferroelectric Memories 19, 400 (1989).
P. Lesaicherre, S. Yamamichi, K. Takemura, H. Yamaguchi, K. Tokashiki, Y. Miyasaka, M. Yoshida, and H. Ono, Intergrated Ferroelectrics 11, 81 (1995).
P. Muralt, A. Kholkin, M. Kohli, T. Maeder, K.G. Brooks, and R. Luthier, Intergrated Ferroelectrics 11, 213 (1995).
R.W. Whatmore, P. Kirby, A. Patel, N.M. Shorrocks, T. Bland, and M. Walker, Science and Technology of Electroceramic Thin Films, 383 (1995).
G. Teowee and C.D. Baetlein, Intergrated Ferroelectrics 11, 47 (1995).
S.J. Chae, S.S. Park, and S.G. Yoon, Intergrated Ferroelectrics 10, 63 (1995).
D.A. Tossell, N.M. Shorrocks, and R.W. Whatmore, Intergrated Ferroelectrics 3, 301 (1993).
C.J. Brierlcy, C. Trundle, L. Considine, R.W. Whatmore, and F.W. Ainger, Ferroelectrics 91, 181 (1989).
A.S. Nickles, R. Ramesh, R.M. White, and E.E. Haller, Integrated Ferroelectrics 10, 89 (1995).
R.W. Schwartz, T.J. Boyle, S.J. Lockwood, M.B. Sinclair, D. Dimos, and C.D. Bucheit, Intergrated Ferroelectrics 7, 259 (1995).
M. Klee, R. Eusemann, R. Waser, and W. Brand, J. Appl. Phys. 72(4), 1566 (1992).
Ferroelectricity Newsletter, 3 (Fall 1996).
R.W. Schwartz, R.A. Assink, and T.J. Headley, in Ferroelectric Thin Films II Symposium, edited by A.I. Kingon, E.R. Myers, and B.A. Tuttle (Materials Research Society, Pittsburgh, PA, 1992), vol. 243, p. 345.
U. Kolb, I. Abraham, D. Gutwerk, T.S. Ertel, W. Hörner, H. Bertagnolli, S. Merklein, and D. Sporn, Physica B 208/209, 601 (1995).
P.R. Coffman and S.K. Dey, J. Sol-Gel Sci. Tech. 1, 251 (1994).
S.R. Gurkovich and J.B. Blum, in Ultrastructure Processing of Glasses, Ceramic and Composite, edited by L.L. Hench and D.R. Ulrich (John Wiley, New York, 1984), p. 152.
K.D. Budd, S.K. Dey, and D.A. Payne, Br. Ceram. Proc. 36, 107 (1985).
G. Yi, Z. Wu, and M. Sayer, J. Appl. Phys. 75(5), 2717 (1988).
K.C. Chen, A. Janah, and J.D. Mackensie, in Better Ceramics Through Chemistry II, Materials Research Society Symposium Proceedings, edited by C.J. Brinker, D.E. Clark, and D.R. Ulrich (Materials Research Society, Pittsburgh, PA, 1986), vol. 73, p. 731.
C. Hsueh and M.L. Mecartney, J. Mater. Res. 6(10), 2208 (1991).
N.M. Shorrocks, A. Patel, M.J. Walker, and A.D. Parsons, Microelectronic Engineering 29, 59 (1995).
B.E. Novich and T.A. Ring, Clays Clay Miner. 32, 400 (1984).
B.E. Novich and T.A. Ring, J. Chem. Soc., Faraday Trans. 81, 1455 (1985).
J.D.F. Ramsay, R.G. Avery, and P.J. Russel, Radiochim. Acta. 44/45, 119 (1988).
R. Amal, J.R. Coury, J.A. Raper, W.P. Walsh, and T.D. Waite, Colloid Surf. 46, 1 (1990).
U. von Gunten and W. Schneider, J. Colloid Interface Sci. 145, 127 (1991).
A. Ledin, S. Karlsson, and B. Allard, Appl. Geochem. 8, 409 (1993).
M.S. Caceci and A. Billon, Org. Geochem. 15, 335 (1991).
P.M. Reid, A.E. Wilkinson, E. Tipping, and M.N. Jones, J. Soil Sci. 42, 259 (1991).
P.M. Gschwend and M.D. Reynolds, J. Cont. Hydr. 1, 309 (1987).
K.L. Lee, H.A. Hristov, T.Y. Tien, and E. Gulari, J. Mat. Sci. 31, 1341 (1996).
D.W. Schaefer, K.D. Keefer, and C.J. Brinker, Polymer Preprints, Am. Chem. Soc., Div. of Polymer Chem. 24, 239 (1983).
C.J. Brinker, K.D. Keefer, D.W. Schaefer, and C.S. Ashley, J. Non-Cryst. Solids 48, 47 (1982).
C.J. Brinker, K.D. Keefer, D.W. Schaefer, R.A. Assink, B.D. Kay, and C.S. Ashley, J. Non-Cryst. Solids 63, 45 (1984).
J.A. Jutson, R.M. Richardson, S.L. Jones, and C. Norman, Mat. Res. Soc. Symp. Proc. 180, 123 (1990).
H.M. Jang and M.K. Cho, Mat. Res. Soc. Symp. Proc. 361, 403 (1995).
K. Kezuka, Y. Hashihiro, and T. Yamaguchi, J. Am. Ceram. Soc. 72(9), 1660 (1989).
G. Floudas, A. Rizos, W. Brown, and K.L. Ngai, Macromolecules 27, 2719 (1994).
Ira N. Levine, Physical Chemistry, 3rd edition (Mcgraw-Hill Book Company, Singapore, 1988), p. 486.
O. Glatter and O. Kratky, Small Angle X-ray Scattering (Academic Press, London, 1982).
M.L. Huggins, J. Am. Chem. Soc. 64, 2716 (1942).
A. Einstein, Ann. Phys. 19, 289 (1906).
C.G. Vonk, J. Appl Cryst. 8, 341 (1975).
R.K. Iler, Chemistry of Silica (Wiley, New York, 1979), p. 345.
R.W. Schwartz, B.C. Bunker, D.B. Dimos, R.A. Assink, B.A Tuttle, D.R. Tallant, and I.A. Weinstock, Integrated Ferroelectrics 2, 243 (1992).
S. Doeuff, M. Henry, C. Sanchez, and J. Livage, J. Non-Cryst. Solids 89, 206 (1987).
S. Doeuff, M. Henry, and C. Sanchez, Mat. Res. Bull. 25, 1519 (1990).
S. Doeuff, Y. Dromzee, F. Taulelle, and C. Sanchez, Inorg. Chem. 28, 4439 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, Q., Vickers, M., Patel, A. et al. Determination of Particle Size and Shape during the Hydrolysis of Pb(Zr0.3Ti0.7)O3 Precursor Solutions. Journal of Sol-Gel Science and Technology 11, 141–152 (1998). https://doi.org/10.1023/A:1008641429895
Issue Date:
DOI: https://doi.org/10.1023/A:1008641429895