Abstract
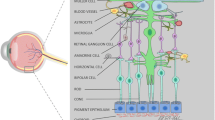
The distribution of mitochondria within retinal glial (Muller) cells and neurons was studied by electron microscopy, by confocal microscopy of a mitochondrial dye and by immunocytochemical demonstration of the mitochondrial enzyme GABA transaminase (GABA-T). We studied sections and enzymatically dissociated cells from adult vascularized (human, pig and rat) and avascular or pseudangiotic (guinea-pig and rabbit) mammalian retinae. The following main observations were made. (1) Muller cells in adult euangiotic (totally vascularized) retinae contain mitochondria throughout their length. (2) Muller cells from the periphery of avascular retinae display mitochondria only within the sclerad-most end of Muller cell processes. (3) Muller cells from the vascularized retinal rim around the optic nerve head in guinea-pigs contain mitochondria throughout their length. (4) Muller cells from the peripapillar myelinated region (‘medullary rays’) of the pseudangiotic rabbit retina contain mitochondria up to their soma. In living dissociated Muller cells from guinea-pig retina, there was no indication of low intracellular pH where the mitochondria were clustered. These data support the hypothesis that Muller cells display mitochondria only at locations of their cytoplasm where the local O2 pressure (pO2) exceeds a certain threshold. In contrast, retinal ganglion cells of guinea-pig and rabbit retinae display many mitochondria although the local pO2 in the inner (vitread) retinal layers has been reported to be extremely low. It is probable that the alignment of mitochondria and the expression of mitochondrial enzymes are regulated by different mechanisms in various types of retinal neurons and glial cells.
Similar content being viewed by others
References
Ahmed, J., Braun, R. D., Dunn, R., Jr & Linsenmeier, R. A. (1993) Oxygen distribution in the macaque retina. Investigative Ophthalmology and Visual Science 34, 516–521.
Alder, V. A., Cringle, S. J. & Constable, I. J. (1983) The retinal oxygen profile in cats. Investigative Ophthalmology and Visual Science 24, 30–36.
Ames A., III (1992) Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Canadian Journal of Physiology and Pharmacology 70, S158–S264.
Bereiter-Hahn, J. & VÖth, M. (1994) Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microscopy Research and Technique 27, 198–219.
Biedermann, B., Eberhardt, W. & Reichelt, W. (1994) GABA uptake into isolated retinal Müller glial cells of the guinea-pig detected electrophysiologically. NeuroReport 5, 438–440.
Buttery, R. G., Hinrichsen, C. F. L., Weller, W. L. & Haight, J. R. (1991) How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vision Research 31, 169–187.
Chase, J. (1982) The evolution of retinal vascularization in mammals. A comparison of vascular and avascular retinae. Ophthalmology 89, 1518–1525.
Eichner, D. & Themann, H. (1962) Zur Frage des Netzhautglykogens beim Meerschweinchen. Zeitschrift für Zellforschung und Mikroskopische Anatomie 56, 231–246.
Germer, A., Wolburg, H., Kuhrt, H., Mack, A. F. & Reichenbach, A. (1998) Distribution of mitochondria within Muüller cells – II. Post-natal development of the rabbit retinal periphery in vivo and in vitro: dependence on oxygen supply. Journal of Neurocytology 27.
Hayashi, T. & Negishi, K. (1981) Histochemical localization of aminobutyric acid transaminase activity in the carp retina. Journal of the Juzen Medical Society 90, 501–507.
Hyde, J. C. & Robinson, N. (1974) Localisation of sites of GABA catabolism in the rat retina. Nature 248, 432–433.
HyvÄrinen, L. (1967) Circulation in the fundus of the rabbit eye. Acta Ophthalmology 45, 862–875.
Johnson, G. L. (1901) Contributions to the comparative anatomy of vertebrates, chiefly based on ophthalmoscopic examination. Philosophical Transactions of the Royal Society of London B 194, 1–82.
Johnson, G. L. (1968) Ophthalmoscopic studies on the eyes of mammals. Philosophical Transactions of the Royal Society of London B 254, 207–220.
Jones, D. P., & Aw, T. Y. (1988) Mitochondrial distribution and O2 gradients in mammalian cells. In Microcompartmentation. (edited by Jones, D. P.) pp. 37–54. Boca Raton, FL: CRC Press.
Krebs, H. A. (1972) The Pasteur effect and the relations between respiration and fermentation. In Essays in Biochemistry, Vol. 8 (edited by Dickens, F. & Campbell, P. N.) pp. 1–34. New York: Academic Press.
Kuriyama, A. K., Sisken, B., Haber, B. & Roberts, E. (1968) The γ-aminobutyric acid system in rabbit retina. Brain Research 9, 165–168.
Kuwabara, T. & Cogan, D. G. (1960) Studies of retinal vascular patterns. I. Normal architecture. Archives of Ophthalmology 64, 904–911.
Lin, C.-T., Li, H.-Z. & Wu, J.-Y. (1983) Immunocytochemical localization of L-glutamate decarboxylase, gammaaminobutyric acid transaminase, cysteine sulfinic acid decarboxylase, aspartate aminotransferase and somatostatin in rat retina. Brain Research 270, 273–283.
Linsenmeier, R. A. (1986) Effects of light and darkness on oxygen distribution and consumption in the cat retina. Journal of General Physiology 88, 521–542.
Lopez-Escalera, R., Li, X.-B., Szerencsei, T. & Schnetkamp, P. M. (1991) Glycolysis and glucose uptake in intact outer segments isolated from bovine retinal rods. Biochemistry 30, 8970–8976.
Mcgeer, P. L. & Mcgeer, E. G. (1989) Amino acid neurotransmitters. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects (edited by Siegel, G., Agranoff, J. B., Albers, R. W. & Molinoff, P.) pp. 311–332. New York: Raven Press.
Marshall, J. & Voaden, M. (1975) Autoradiographic identification of the cells accumulating 3H-aminobutyric acid in mammalian retinae: a species comparison. Vison Research 15, 459–461.
Michaelson, I. C. (1953) Retinal circulation in man and animals. Springfield, IL: Charles C. Thomas.
Neal, M. J., Cunningham, J. R., Shah, M. A. & Yazulla, S. (1989) Immunocytochemical evidence that vigabatrin in rats causes GABA accumulation in glial cells of the retina. Neuroscience Letters 98, 29–32.
Newman, E. A. (1996) Acid efflux from retinal glial cells generated by sodium bicarbonate cotransport. Journal of Neuroscience 16, 159–168.
Penn, R. D. & Hagins, W. A. (1972) Kinetics of the photocurrent of retinal rods. Biophysical Journal 12, 1073–1094.
Peters, A., Palay, S. L. & Webster, H. (1991) The Fine Structure of the Nervous System. Neurons and their Supporting Cells. 3rd edn. New York, Oxford: Oxford University Press.
Poitry-Yamate, C. L., Poitry, S. & Tsacopoulos, M. (1995) Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. Journal of Neuroscience 15, 5179–5191.
Poot, M., Zhang, Y. Z., Kramer, J. A., Wells, K. S., Jones, L. J., Hanzel, D. K., Lugade, A. G., Singer, V. L. & Haugland, R. P. (1996) Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. Journal of Histochemistry and Cytochemistry 44, 1363–1372.
Pournaras, C. J., Riva, E. C., Tsacopoulos, M. & Strommer, K. (1989) Diffusion of O2 in the retina of anaesthetized miniature pigs in normoxia and hyperoxia. Experimental Eye Research 49, 347–360.
Pow, D. V. & Robinson, S. R. (1994) Glutamate in some retinal neurons is derived solely from glia. Neuroscience 60, 355–366.
Pow, D. V. and Rogers, P. (1996) GABA transamination regulates neuronal glutamate content in the retina. NeuroReport 7, 2683–2686.
Rasmussen, K. E. (1973) A morphometric study of the Muüller cells, their nuclei and mitochondria, in the rat retina. Journal of Ultrastructure Research 44, 96–112.
Reichenbach, A. (1989) Organelle-free cytoplasmic volume fraction of rabbit retinal Muüller (glial) cells. Journal für Hirnforschung 30, 513–516.
Reichenbach, A., Hagen, E., Schippel, K. & Eberhardt, W. (1988a) Quantitative electron microscopy of rabbit Müller (glial) cells in dependence of retinal topography. Zeitschrift für Mikroskopisch-anatomische Forschung 102, 721–755.
Reichenbach, A., Hagen, E., Schippel, K., BrÜckner, G., Reichelt, W. & Leibnitz, L. (1988b) Cytotopographical specialization of enzymatically isolated rabbit retinal Müller (glial) cells. Structure, ultrastructure, and (3)H-ouabain binding sites. Zeitschrift für Mikroskopisch-anatatomische Forschung 102, 897–912.
Reichenbach, A., Wolburg, H., Richter, W. & Eberhardt, W. (1990) Membrane ultrastructure preservation and membrane potentials after isolation of rabbit retinal glial (Müller) cells by papain. Journal of Neuroscience Methods 32, 227–233.
Reichenbach, A., Stolzenburg, J.-U., Eberhardt, W., Chao, T. I., Dettmer, D. & Hertz, L. (1993) What do retinal Müller (glial) cells do for their neuronal "small siblings’. Journal of Neurochemical Anatomy 6, 201–213.
Rohen, J. (1954) Über das Gefäβsystem der Retina beimKaninchen. Ophthalmologica 128, 307–317.
Schousboe, I., Bro, B. & Schousboe, A. (1977) Intramitochondrial localization of the 4-aminobutyrate-2-oxoglutarate transaminase from ox brain. Biochemical Journal 162, 303–307.
Stirling, C. E. & Sarthy, P. V. (1985) Localization of the Na-K pump in turtle retina. Journal of Neurocytology 14, 33–47.
Tillis, T. N., Murray, D. L., Schmidt, G. J. & Weiter, J. J. (1988) Preretinal oxygen changes in the rabbit under conditions of light and dark. Investigative Ophthalmology and Visual Science 29, 988–991.
Uga, S. & Smelser, G. K. (1973) Comparative study of the fine structure of retinal Müller cells in various vertebrates. Investigative Ophthalmology and Visual Science 12, 434–448.
Wang, L., Kondo, M. & Bill, A. (1997) Glucose metabolism in cat outer retina. Effects of light and hyperoxia. Investigative Ophthalmology and Visual Science 38, 48–55.
Winkler, B. S. (1995) A quantitative assessment of glucose metabolism in the isolated rat retina. In Vision et Adaptation, Vol. 6 (edited by Christen, Y., Doly, M. and Droy-Lefaix, M.-T.) pp. 78–96. Amsterdam: Elsevier.
Yamamoto, F. & Steinberg, R. H. (1992) Effects of systemic hypoxia on pH outside rod photoreceptors in the cat retina. Experimental Eye Research 54, 699–709.
Yu, D.-Y., Cringle, S. J., Alder, V. A., Su, E.-N. & Yu, P. K. (1994) Intraretinal oxygen distribution in rats as a function of systemic blood pressure. American Journal of Physiology 36, H2498–H2507.
Yu, D.-Y., Cringle, S. J., Alder, V. A., Su, E.-N. & Yu, P. K. (1996) Intraretinal oxygen distribution and choroidal regulation in the avascular retina of guinea pig. American Journal of Physiology 270, H965–H973.
Zuckerman, R. & Weiter, J. J. (1980) Oxygen transport in the bullfrog retina. Experimental Eye Research 30, 117–127.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Germer, A., Biedermann, B., Wolburg, H. et al. Distribution of mitochondria within Muller cells – I. Correlation with retinal vascularization in different mammalian species. J Neurocytol 27, 329–345 (1998). https://doi.org/10.1023/A:1006934724566
Issue Date:
DOI: https://doi.org/10.1023/A:1006934724566